当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective direct Mannich-type reactions of 2-benzylpyridine N-oxides catalyzed by chiral bis(guanidino)iminophosphorane organosuperbase†
Chemical Science ( IF 7.6 ) Pub Date : 2018-04-20 00:00:00 , DOI: 10.1039/c8sc00808f Qiupeng Hu 1 , Azusa Kondoh 2 , Masahiro Terada 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-04-20 00:00:00 , DOI: 10.1039/c8sc00808f Qiupeng Hu 1 , Azusa Kondoh 2 , Masahiro Terada 1
Affiliation
|
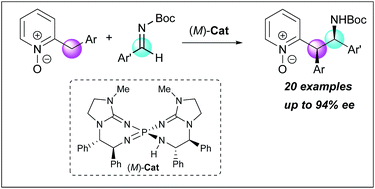
2-Benzylpyridine N-oxides possessing less acidic α-protons were utilized as pronucleophiles for the first time in enantioselective addition reactions under Brønsted base catalysis. A chiral bis(guanidino)iminophosphorane was able to overcome the inherent issue of low acidity of the pronucleophiles, establishing the diastereo- and enantioselective direct Mannich-type reaction with N-Boc imines. The control experiments indicated that the N-oxide moiety of the substrates played a critical role in achieving the high stereoselectivity.
中文翻译:

手性双(胍基)亚氨基正膦有机超碱催化的 2-苄基吡啶 N-氧化物的对映选择性直接曼尼希型反应†
具有较少酸性α-质子的2-苄基吡啶N-氧化物首次被用作亲核试剂,用于布朗斯台德碱催化下的对映选择性加成反应。手性双(胍基)亚氨基正膦能够克服亲核试剂低酸性的固有问题,与N -Boc亚胺建立非对映和对映选择性直接曼尼希型反应。对照实验表明底物的N-氧化物部分在实现高立体选择性方面发挥着关键作用。
更新日期:2018-04-20
中文翻译:

手性双(胍基)亚氨基正膦有机超碱催化的 2-苄基吡啶 N-氧化物的对映选择性直接曼尼希型反应†
具有较少酸性α-质子的2-苄基吡啶N-氧化物首次被用作亲核试剂,用于布朗斯台德碱催化下的对映选择性加成反应。手性双(胍基)亚氨基正膦能够克服亲核试剂低酸性的固有问题,与N -Boc亚胺建立非对映和对映选择性直接曼尼希型反应。对照实验表明底物的N-氧化物部分在实现高立体选择性方面发挥着关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号