当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reductive coupling of benzyl oxalates with highly functionalized alkyl bromides by nickel catalysis†
Chemical Science ( IF 7.6 ) Pub Date : 2018-04-20 00:00:00 , DOI: 10.1039/c8sc00609a Xiao-Biao Yan 1 , Chun-Ling Li 1 , Wen-Jie Jin 1 , Peng Guo 1 , Xing-Zhong Shu 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-04-20 00:00:00 , DOI: 10.1039/c8sc00609a Xiao-Biao Yan 1 , Chun-Ling Li 1 , Wen-Jie Jin 1 , Peng Guo 1 , Xing-Zhong Shu 1
Affiliation
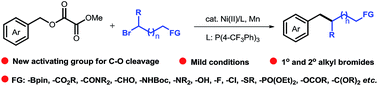
|
Coupling reactions involving non-sulfonated C–O electrophiles provide a promising method for forming C–C bonds, but the incorporation of functionalized or secondary alkyl groups remains a challenge due to the requirement for well-defined alkylmetal species. In this study, we report a reductive nickel-catalyzed cross-coupling of benzyl oxalates with alkyl bromides, using oxalate as a new leaving group. A broad range of highly functionalized alkyl units (such as functional groups: alkyl chloride, alcohol, aldehyde, amine, amide, boronate ester, ether, ester, heterocycle, phosphonate, strained ring) were efficiently incorporated at the benzylic position. The utility of this synthetic method was further demonstrated by late-stage modification of complex bioactive compounds. Preliminary mechanistic experiments revealed that a radical process might be involved in the reaction.
中文翻译:

通过镍催化将草酸苄酯与高度官能化的烷基溴还原偶联†
涉及非磺化C-O亲电子试剂的偶联反应为形成C-C键提供了一种有前途的方法,但由于需要明确的烷基金属种类,官能化或仲烷基的掺入仍然是一个挑战。在这项研究中,我们报道了草酸苄酯与烷基溴的还原镍催化交叉偶联,使用草酸作为新的离去基团。各种高度官能化的烷基单元(例如官能团:烷基氯、醇、醛、胺、酰胺、硼酸酯、醚、酯、杂环、膦酸酯、张力环)被有效地结合在苄基位置。通过复杂生物活性化合物的后期修饰进一步证明了这种合成方法的实用性。初步的机理实验表明,该反应可能涉及自由基过程。
更新日期:2018-04-20
中文翻译:

通过镍催化将草酸苄酯与高度官能化的烷基溴还原偶联†
涉及非磺化C-O亲电子试剂的偶联反应为形成C-C键提供了一种有前途的方法,但由于需要明确的烷基金属种类,官能化或仲烷基的掺入仍然是一个挑战。在这项研究中,我们报道了草酸苄酯与烷基溴的还原镍催化交叉偶联,使用草酸作为新的离去基团。各种高度官能化的烷基单元(例如官能团:烷基氯、醇、醛、胺、酰胺、硼酸酯、醚、酯、杂环、膦酸酯、张力环)被有效地结合在苄基位置。通过复杂生物活性化合物的后期修饰进一步证明了这种合成方法的实用性。初步的机理实验表明,该反应可能涉及自由基过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号