当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluating Water Reactivity at Silica Surfaces Using Reactive Potentials
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jpcc.7b12653 Thiruvilla S. Mahadevan 1 , Jincheng Du 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jpcc.7b12653 Thiruvilla S. Mahadevan 1 , Jincheng Du 1
Affiliation
|
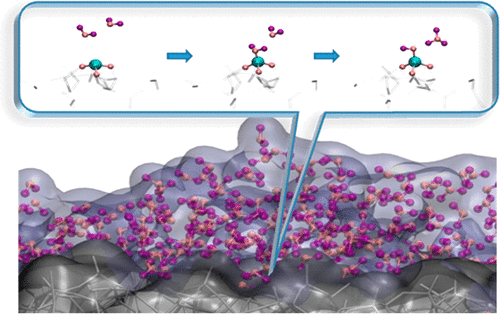
Understanding the interactions between amorphous silica surfaces and water provides insight into material degradation of silicate glasses and minerals in aqueous environment. Molecular dynamics (MD) simulations of water and nanometer sized silica structures were used in this work to evaluate the reactivity of flat silica surface and surfaces with curvature. We compared two dissociable water/silica potentials, namely the Reactive Force Field (ReaxFF) and the Mahadevan–Garofalini water/silica force field (MGFF) that have been in development over the past decade, to study their performance in simulating bulk water as well as silica–water interactions. Significant differences in the properties of bulk water as well as surface interactions were observed between the two types of potentials, as well in the same potential type with two parametrizations for ReaxFF, suggesting a need for improvement of the existing water/silica ReaxFF potentials. Our simulation results show that a majority of the silanols were formed by reactions between water and strained siloxane bonds that mainly exist on the surface of amorphous silica, within a few nanoseconds of the simulation time scale, in agreement with previous studies. Effect of surface curvature on the reactivity with water was investigated. Our results indicate that defect concentration at the surface bears a strong correlation to the concentration of silanols (Si–OH) that eventually form. We observe undercoordinated Si’s at the surface that are attacked by water before the hydrolysis reaction of the siloxane bonds and demonstrate possible mechanisms of water reacting with these undercoordinated Si’s. We also find that the method of generating surfaces in simulation determines the defect concentration and hence influences the reactivity of the amorphous silica surface.
中文翻译:

使用反应电势评估二氧化硅表面的水反应性
了解无定形二氧化硅表面与水之间的相互作用可深入了解硅酸盐玻璃和矿物质在水性环境中的材料降解。水和纳米级二氧化硅结构的分子动力学(MD)模拟用于这项工作,以评估平坦的二氧化硅表面和具有曲率的表面的反应性。我们比较了过去十年来一直在开发的两个可分解的水/二氧化硅电势,即反作用力场(ReaxFF)和马哈德万-加洛法利尼水/二氧化硅力场(MGFF),还研究了它们在模拟散装水方面的性能作为二氧化硅与水的相互作用。两种电位之间在散装水的性质以及表面相互作用方面存在显着差异,以及具有ReaxFF的两个参数化的相同电势类型,这表明需要改进现有的水/硅ReaxFF电势。我们的模拟结果表明,大多数硅烷醇是由水和应变硅氧烷键之间的反应形成的,而这些键主要存在于无定形二氧化硅表面上,在模拟时间尺度的几纳秒内,与先前的研究一致。研究了表面曲率对与水反应性的影响。我们的结果表明,表面缺陷浓度与最终形成的硅烷醇(Si-OH)浓度密切相关。我们观察到在硅氧烷键的水解反应之前受水侵蚀的表面配位不足的Si,并证明了水与这些配位不足的Si反应的可能机理。我们还发现,模拟中生成表面的方法确定了缺陷浓度,因此影响了无定形二氧化硅表面的反应性。
更新日期:2018-04-19
中文翻译:

使用反应电势评估二氧化硅表面的水反应性
了解无定形二氧化硅表面与水之间的相互作用可深入了解硅酸盐玻璃和矿物质在水性环境中的材料降解。水和纳米级二氧化硅结构的分子动力学(MD)模拟用于这项工作,以评估平坦的二氧化硅表面和具有曲率的表面的反应性。我们比较了过去十年来一直在开发的两个可分解的水/二氧化硅电势,即反作用力场(ReaxFF)和马哈德万-加洛法利尼水/二氧化硅力场(MGFF),还研究了它们在模拟散装水方面的性能作为二氧化硅与水的相互作用。两种电位之间在散装水的性质以及表面相互作用方面存在显着差异,以及具有ReaxFF的两个参数化的相同电势类型,这表明需要改进现有的水/硅ReaxFF电势。我们的模拟结果表明,大多数硅烷醇是由水和应变硅氧烷键之间的反应形成的,而这些键主要存在于无定形二氧化硅表面上,在模拟时间尺度的几纳秒内,与先前的研究一致。研究了表面曲率对与水反应性的影响。我们的结果表明,表面缺陷浓度与最终形成的硅烷醇(Si-OH)浓度密切相关。我们观察到在硅氧烷键的水解反应之前受水侵蚀的表面配位不足的Si,并证明了水与这些配位不足的Si反应的可能机理。我们还发现,模拟中生成表面的方法确定了缺陷浓度,因此影响了无定形二氧化硅表面的反应性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号