当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Interaction of CO2 with CeO2 Powder Explored by Correlating Adsorption and Thermal Desorption Analyses
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jpcc.8b01299 Danielle Schweke 1 , Shimon Zalkind 1 , Smadar Attia 2 , Joseph Bloch 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jpcc.8b01299 Danielle Schweke 1 , Shimon Zalkind 1 , Smadar Attia 2 , Joseph Bloch 1
Affiliation
|
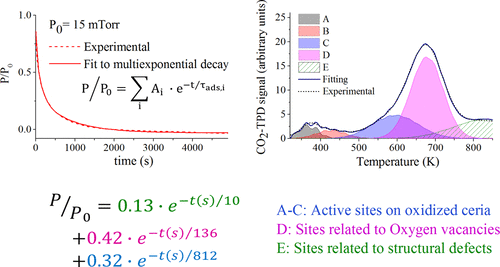
Understanding the interplay between thermodynamics and kinetics is of high importance for the optimization of catalytic reactions involving the adsorption of CO2 on CeO2 (ceria). The present study explores the interaction of CO2 with ceria powder in near-realistic conditions by correlating adsorption and thermal desorption analyses. Activation energies for desorption, Ea, and kinetic parameters (adsorption time constants, τ, and sticking coefficients, s0) are determined using a new methodology based on surface science models. The sticking coefficients obtained for CO2 on ceria powder are significantly lower than those observed for CO2 on flat surfaces. CO2 is found to adsorb most rapidly on sites attributed to surface defects. CO2 adsorption is slower on nondefective active sites, leading to the formation of various carbonate species. The desorption analysis indicates that each peak in the CO2-TPD profiles is composed of several subpeaks, resulting from various binding sites for CO2 on the polycrystalline powder. The distribution of the chemisorbed CO2 species between the different sites, the corresponding adsorption energies, and the influence of coverage on those energies are thus determined. In addition, the correlation between adsorption and desorption analyses indicates the influence of heating on the distribution of the chemisorbed species.
中文翻译:

吸附与热解吸相关性探讨CO 2与CeO 2粉末的相互作用
了解热力学与动力学之间的相互作用对于优化涉及CeO 2(二氧化铈)上的CO 2吸附的催化反应非常重要。本研究通过关联吸附和热脱附分析,探索了在接近实际条件下CO 2与二氧化铈粉末的相互作用。使用基于表面科学模型的新方法确定解吸的活化能E a和动力学参数(吸附时间常数τ和粘着系数s 0)。在二氧化铈粉末上获得的CO 2粘附系数显着低于CO 2所观察到的粘附系数在平坦的表面上。发现CO 2最迅速地吸附在归因于表面缺陷的部位上。在无缺陷的活性位点上,CO 2吸附较慢,导致形成各种碳酸盐物质。解吸分析表明,CO 2 -TPD曲线中的每个峰均由多个亚峰组成,这是由多晶粉末上CO 2的各种结合位点引起的。化学吸附CO 2的分布因此确定了不同位点之间的分子种类,相应的吸附能以及覆盖度对那些能的影响。另外,吸附和解吸分析之间的相关性表明加热对化学吸附物质分布的影响。
更新日期:2018-04-19
中文翻译:

吸附与热解吸相关性探讨CO 2与CeO 2粉末的相互作用
了解热力学与动力学之间的相互作用对于优化涉及CeO 2(二氧化铈)上的CO 2吸附的催化反应非常重要。本研究通过关联吸附和热脱附分析,探索了在接近实际条件下CO 2与二氧化铈粉末的相互作用。使用基于表面科学模型的新方法确定解吸的活化能E a和动力学参数(吸附时间常数τ和粘着系数s 0)。在二氧化铈粉末上获得的CO 2粘附系数显着低于CO 2所观察到的粘附系数在平坦的表面上。发现CO 2最迅速地吸附在归因于表面缺陷的部位上。在无缺陷的活性位点上,CO 2吸附较慢,导致形成各种碳酸盐物质。解吸分析表明,CO 2 -TPD曲线中的每个峰均由多个亚峰组成,这是由多晶粉末上CO 2的各种结合位点引起的。化学吸附CO 2的分布因此确定了不同位点之间的分子种类,相应的吸附能以及覆盖度对那些能的影响。另外,吸附和解吸分析之间的相关性表明加热对化学吸附物质分布的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号