当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Foldamer Tertiary Structure through Sequence-Guided Protein Backbone Alteration
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.accounts.8b00048 Kelly L George 1 , W Seth Horne 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.accounts.8b00048 Kelly L George 1 , W Seth Horne 1
Affiliation
|
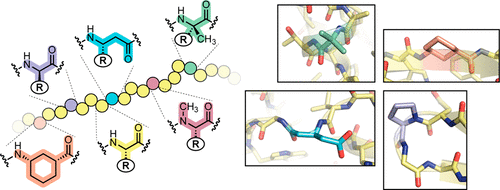
The prospect of recreating the complex structural hierarchy of protein folding in synthetic oligomers with backbones that are artificial in covalent structure (“foldamers”) has long fascinated chemists. Foldamers offer complex functions from biostable scaffolds and have found widespread applications in fields from biomedical to materials science. Most precedent has focused on isolated secondary structures or their assemblies. In considering the goal of complex protein-like tertiary folding patterns, a key barrier became apparent. How does one design a backbone with covalent connectivity and a sequence of side-chain functional groups that will support defined intramolecular packing of multiple artificial secondary structures? Two developments were key to overcoming this challenge. First was the recognition of the power of blending α-amino acid residues with monomers differing in backbone connectivity to create “heterogeneous-backbone” foldamers. Second was the finding that replacing some of the natural α-residues in a biological sequence with artificial-backbone variants can result in a mimic that retains both the fold and function of the native sequence and, in some cases, gains advantageous characteristics. Taken together, these precedents lead to a view of a protein as chemical entity having two orthogonal sequences: a sequence of side-chain functional groups and a separate sequence of backbone units displaying those functional groups.
中文翻译:

通过序列引导的蛋白质主链改变的 Foldamer 三级结构
在具有共价结构的人工主链(“折叠体”)的合成低聚物中重建蛋白质折叠的复杂结构层次的前景长期以来一直令化学家着迷。折叠分子通过生物稳定支架提供复杂的功能,并在生物医学到材料科学等领域得到广泛应用。大多数先例都集中在孤立的二级结构或其组件上。在考虑复杂的类蛋白质三级折叠模式的目标时,一个关键障碍变得明显。如何设计具有共价连接性和一系列侧链官能团的主链,以支持多个人工二级结构的确定的分子内堆积?两项发展是克服这一挑战的关键。首先是认识到将 α-氨基酸残基与主链连接性不同的单体混合以创建“异质主链”折叠体的能力。其次,我们发现用人工主链变体替换生物序列中的一些天然 α 残基可以产生保留天然序列的折叠和功能的模拟物,并且在某些情况下获得有利的特性。总而言之,这些先例导致了将蛋白质视为具有两个正交序列的化学实体的观点:侧链官能团的序列和显示这些官能团的主链单元的单独序列。
更新日期:2018-04-19
中文翻译:

通过序列引导的蛋白质主链改变的 Foldamer 三级结构
在具有共价结构的人工主链(“折叠体”)的合成低聚物中重建蛋白质折叠的复杂结构层次的前景长期以来一直令化学家着迷。折叠分子通过生物稳定支架提供复杂的功能,并在生物医学到材料科学等领域得到广泛应用。大多数先例都集中在孤立的二级结构或其组件上。在考虑复杂的类蛋白质三级折叠模式的目标时,一个关键障碍变得明显。如何设计具有共价连接性和一系列侧链官能团的主链,以支持多个人工二级结构的确定的分子内堆积?两项发展是克服这一挑战的关键。首先是认识到将 α-氨基酸残基与主链连接性不同的单体混合以创建“异质主链”折叠体的能力。其次,我们发现用人工主链变体替换生物序列中的一些天然 α 残基可以产生保留天然序列的折叠和功能的模拟物,并且在某些情况下获得有利的特性。总而言之,这些先例导致了将蛋白质视为具有两个正交序列的化学实体的观点:侧链官能团的序列和显示这些官能团的主链单元的单独序列。











































 京公网安备 11010802027423号
京公网安备 11010802027423号