当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conjugate Addition−Enantioselective Protonation of N-Aryl Glycines to α-Branched 2-Vinylazaarenes via Cooperative Photoredox and Asymmetric Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-04-10 , DOI: 10.1021/jacs.8b01575 Yanli Yin 1, 2 , Yating Dai 1 , Hongshao Jia 1 , Jiangtao Li 1 , Liwei Bu 1 , Baokun Qiao 1 , Xiaowei Zhao 1 , Zhiyong Jiang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-04-10 , DOI: 10.1021/jacs.8b01575 Yanli Yin 1, 2 , Yating Dai 1 , Hongshao Jia 1 , Jiangtao Li 1 , Liwei Bu 1 , Baokun Qiao 1 , Xiaowei Zhao 1 , Zhiyong Jiang 1
Affiliation
|
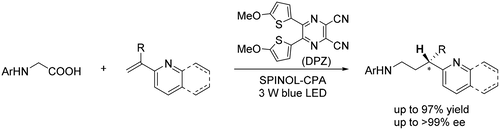
An enantioselective protonation strategy has been successfully applied to the synthesis of chiral α-tertiary azaarenes. With a dual catalytic system involving a chiral phosphoric acid and a dicyanopyrazine-derived chromophore (DPZ) photosensitizer that is mediated by visible light, a variety of α-branched 2-vinylpyridines and 2-vinylquinolines with N-aryl glycines underwent a redox-neutral, radical conjugate addition-protonation process and provided valuable chiral 3-(2-pyridine/quinoline)-3-substituted amines in high yields with good to excellent enantioselectivities (up to >99% ee). An application of this methodology to a two-step synthesis of the enantiomerically pure medicinal compound pheniramine (Avil) is also presented.
中文翻译:

N-芳基甘氨酸通过协同光氧化还原和不对称催化共轭加成-对映选择性质子化为α-支链2-乙烯基氮杂芳烃
对映选择性质子化策略已成功应用于手性α-叔氮杂芳烃的合成。使用由可见光介导的手性磷酸和二氰基吡嗪衍生的发色团 (DPZ) 光敏剂的双重催化系统,各种 α-支链 2-乙烯基吡啶和 2-乙烯基喹啉与 N-芳基甘氨酸发生氧化还原反应,自由基共轭加成 - 质子化过程,并以高产率提供有价值的手性 3-(2-吡啶/喹啉)-3-取代胺,具有良好到出色的对映选择性(高达> 99%ee)。还介绍了该方法在对映异构纯药用化合物苯那敏 (Avil) 的两步合成中的应用。
更新日期:2018-04-10
中文翻译:

N-芳基甘氨酸通过协同光氧化还原和不对称催化共轭加成-对映选择性质子化为α-支链2-乙烯基氮杂芳烃
对映选择性质子化策略已成功应用于手性α-叔氮杂芳烃的合成。使用由可见光介导的手性磷酸和二氰基吡嗪衍生的发色团 (DPZ) 光敏剂的双重催化系统,各种 α-支链 2-乙烯基吡啶和 2-乙烯基喹啉与 N-芳基甘氨酸发生氧化还原反应,自由基共轭加成 - 质子化过程,并以高产率提供有价值的手性 3-(2-吡啶/喹啉)-3-取代胺,具有良好到出色的对映选择性(高达> 99%ee)。还介绍了该方法在对映异构纯药用化合物苯那敏 (Avil) 的两步合成中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号