当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Absorbed hydrogen enhances the catalytic hydrogenation activity of Rh-based nanocatalysts†
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1039/c8cy00522b Franck Morfin 1, 2, 3, 4, 5 , Lucie Blondeau 3, 6, 7, 8, 9 , Karine Provost 3, 6, 7, 8, 9 , Abdelmalek Malouche 3, 6, 7, 8, 9 , Laurent Piccolo 1, 2, 3, 4, 5 , Claudia Zlotea 3, 6, 7, 8, 9
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1039/c8cy00522b Franck Morfin 1, 2, 3, 4, 5 , Lucie Blondeau 3, 6, 7, 8, 9 , Karine Provost 3, 6, 7, 8, 9 , Abdelmalek Malouche 3, 6, 7, 8, 9 , Laurent Piccolo 1, 2, 3, 4, 5 , Claudia Zlotea 3, 6, 7, 8, 9
Affiliation
|
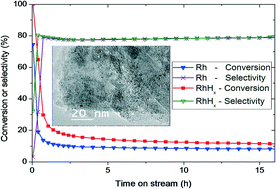
Carbon-supported Rh-based nanoparticles with an average size of 1.0 nm have been tested for the hydrogenation of butadiene in two different forms: either metal Rh or hydride RhHx nanoparticles. Laboratory tests demonstrated that the Rh hydride nanocatalyst is more active than its metal counterpart, irrespective of the gas feed composition and temperature. Moreover, this difference is significantly more important at the initial stage of the reaction compared to that under quasi-stationary conditions. However, the reaction mechanisms appear similar for the metal and hydride nanocatalysts, as suggested by the similar selectivities to butenes, apparent reaction energies, and reaction orders. The local structures of both Rh and RhHx nanocatalysts were studied by operando XAS under stationary conditions. EXAFS analyses confirm that the RhHx and Rh catalysts preserve their structure during the reaction as either the hydride or metal phase, respectively. We suggest that the Mars–van Krevelen mechanism might occur at the initial stage but becomes progressively less predominant. Under quasi-stationary conditions, only electronic effects are invoked to explain the activity difference between the hydride and the metal phases. We hypothesize that the stabilization of Rh–H bonds at the surface in the presence of subsurface hydrogen, consistent with previous theoretical findings, explains the higher activity of the hydride catalyst.
中文翻译:

吸收的氢气增强了Rh基纳米催化剂的催化加氢活性†
已经测试了平均大小为1.0 nm的碳负载Rh基纳米颗粒用于丁二烯氢化的两种不同形式:金属Rh或氢化物RhH x纳米颗粒。实验室测试表明,无论气体进料组成和温度如何,Rh氢化物纳米催化剂都比其金属对应物更具活性。此外,与准静态条件下的反应相比,该差异在反应的初始阶段更为重要。但是,对于丁烯的选择性,表观反应能和反应顺序相似,表明金属和氢化物纳米催化剂的反应机理相似。既Rh和RhH的局部结构X通过研究纳米催化剂operando固定条件下的XAS。EXAFS分析证实,RhH x和Rh催化剂在反应过程中分别以氢化物相或金属相保留其结构。我们建议Mars-van Krevelen机制可能出现在初始阶段,但逐渐变得不那么占主导地位。在准平稳条件下,仅使用电子效应来解释氢化物和金属相之间的活度差异。我们假设,在存在次表面氢的情况下,表面上Rh–H键的稳定性与先前的理论发现一致,这说明了氢化物催化剂的更高活性。
更新日期:2018-04-19
中文翻译:

吸收的氢气增强了Rh基纳米催化剂的催化加氢活性†
已经测试了平均大小为1.0 nm的碳负载Rh基纳米颗粒用于丁二烯氢化的两种不同形式:金属Rh或氢化物RhH x纳米颗粒。实验室测试表明,无论气体进料组成和温度如何,Rh氢化物纳米催化剂都比其金属对应物更具活性。此外,与准静态条件下的反应相比,该差异在反应的初始阶段更为重要。但是,对于丁烯的选择性,表观反应能和反应顺序相似,表明金属和氢化物纳米催化剂的反应机理相似。既Rh和RhH的局部结构X通过研究纳米催化剂operando固定条件下的XAS。EXAFS分析证实,RhH x和Rh催化剂在反应过程中分别以氢化物相或金属相保留其结构。我们建议Mars-van Krevelen机制可能出现在初始阶段,但逐渐变得不那么占主导地位。在准平稳条件下,仅使用电子效应来解释氢化物和金属相之间的活度差异。我们假设,在存在次表面氢的情况下,表面上Rh–H键的稳定性与先前的理论发现一致,这说明了氢化物催化剂的更高活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号