当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Use multiscale simulation to explore the effects of the homodimerizations between different conformation states on the activation and allosteric pathway for the μ-opioid receptor†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1039/c8cp02016g Xi Zhang 1, 2, 3, 4 , Yuan Yuan 5, 6, 7, 8 , Longrong Wang 1, 2, 3, 4 , Yanzhi Guo 1, 2, 3, 4 , Menglong Li 1, 2, 3, 4 , Chuan Li 2, 3, 8, 9 , Xuemei Pu 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1039/c8cp02016g Xi Zhang 1, 2, 3, 4 , Yuan Yuan 5, 6, 7, 8 , Longrong Wang 1, 2, 3, 4 , Yanzhi Guo 1, 2, 3, 4 , Menglong Li 1, 2, 3, 4 , Chuan Li 2, 3, 8, 9 , Xuemei Pu 1, 2, 3, 4
Affiliation
|
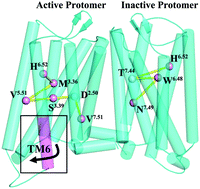
Recently, oligomers of G-protein coupled receptors (GPCRs) have been an important topic in the GPCR fields. However, knowledge about their structures and activation mechanisms is very limited due to the absence of crystal structures reported. In this work, we used multiscale simulations to study the effects of homodimerization between different conformation states on their activation, dynamic behaviors, and allosteric communication pathways for μ-OR. The results indicated that the dimerization of one inactive monomer with either one inactive monomer or one active one could enhance its constitutive activation. However, the conformation state of the other protomer (e.g., active or inactive) can influence the activated extent. The dimerization between the two inactive protomers leads to a negative cooperativity for their activation, which should contribute to the asymmetric activation of GPCR dimers observed in some experiments. On the other hand, for the active monomer, its dimerization with one inactive receptor could alleviate its deactivation, whereby negative and positive cooperativities can be observed between the two subunits of the dimer, depending on the different regions. Observations from protein structure network (PSN) analysis indicated that the dimerization of one inactive monomer with one active one would cause a significant drop in the number of main pathways from the ligand binding pocket to the G-protein coupled region for the inactive protomer, while the impact is minor for the active protomer. But, for the active monomer or the inactive one, its dimerization with one inactive monomer would significantly change the types of residues participating in the pathway with the highest frequency.
中文翻译:

使用多尺度模拟探索不同构象状态之间的同型二聚化对μ阿片受体†的激活和变构途径的影响†
近年来,G蛋白偶联受体(GPCR)的寡聚物已成为GPCR领域的重要课题。然而,由于缺乏所报道的晶体结构,关于它们的结构和活化机理的知识非常有限。在这项工作中,我们使用多尺度模拟研究了不同构象状态之间同源二聚化对其激活,动态行为和μ-OR的变构通讯途径的影响。结果表明,一种惰性单体与一种惰性单体或一种活性单体的二聚化可以增强其组成活化。但是,另一个protomer的构象状态(例如,有效或无效)会影响已激活的范围。两个非活性启动子之间的二聚化导致它们的激活具有负协同性,这应有助于在某些实验中观察到的GPCR二聚体的不对称激活。另一方面,对于活性单体,其与一个非活性受体的二聚化可以减轻其失活,从而可以根据不同区域在二聚体的两个亚基之间观察到负的和正的协同作用。蛋白质结构网络(PSN)分析的观察结果表明,一种无活性单体与一种有活性单体的二聚化将导致从配体结合袋到无活性前列腺素的G蛋白偶联区的主要途径数量显着下降,而对于活动的protomer影响很小。
更新日期:2018-04-19
中文翻译:

使用多尺度模拟探索不同构象状态之间的同型二聚化对μ阿片受体†的激活和变构途径的影响†
近年来,G蛋白偶联受体(GPCR)的寡聚物已成为GPCR领域的重要课题。然而,由于缺乏所报道的晶体结构,关于它们的结构和活化机理的知识非常有限。在这项工作中,我们使用多尺度模拟研究了不同构象状态之间同源二聚化对其激活,动态行为和μ-OR的变构通讯途径的影响。结果表明,一种惰性单体与一种惰性单体或一种活性单体的二聚化可以增强其组成活化。但是,另一个protomer的构象状态(例如,有效或无效)会影响已激活的范围。两个非活性启动子之间的二聚化导致它们的激活具有负协同性,这应有助于在某些实验中观察到的GPCR二聚体的不对称激活。另一方面,对于活性单体,其与一个非活性受体的二聚化可以减轻其失活,从而可以根据不同区域在二聚体的两个亚基之间观察到负的和正的协同作用。蛋白质结构网络(PSN)分析的观察结果表明,一种无活性单体与一种有活性单体的二聚化将导致从配体结合袋到无活性前列腺素的G蛋白偶联区的主要途径数量显着下降,而对于活动的protomer影响很小。











































 京公网安备 11010802027423号
京公网安备 11010802027423号