当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile preparation of a nano-imprinted polymer on magnetite nanoparticles for the rapid separation of lead ions from aqueous solution†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1039/c8cp01163j Delong Kong 1, 2, 3, 4 , Ning Qiao 1, 2, 3, 4 , Nian Wang 1, 2, 3, 4 , Zhuo Wang 1, 2, 3, 4 , Qi Wang 1, 2, 3, 4 , Zhiyong Zhou 1, 2, 3, 4 , Zhongqi Ren 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1039/c8cp01163j Delong Kong 1, 2, 3, 4 , Ning Qiao 1, 2, 3, 4 , Nian Wang 1, 2, 3, 4 , Zhuo Wang 1, 2, 3, 4 , Qi Wang 1, 2, 3, 4 , Zhiyong Zhou 1, 2, 3, 4 , Zhongqi Ren 1, 2, 3, 4
Affiliation
|
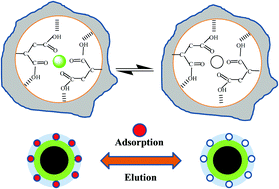
A novel nanostructured magnetic ion-imprinted polymer (IIP) was synthesized for the selective adsorption of Pb(II) from aqueous solution. The IIP was prepared on functional Fe3O4@SiO2 core/shell nanoparticles as a support. Monomer units in the polymer featured the typical bidentate ligand itaconic acid. We used ethylene glycol dimethacrylate and 2,2-azoisobisbutyronitrile as a cross-linker and an initiator, respectively. Monomers with different acid–base properties and different proportions of cross-linker were investigated to obtain high-performance adsorbents. Our results showed that the IIP prepared from itaconic acid had a high adsorption capacity owing to the strong binding between the monomer and Pb(II) template ion. The IIPs were characterized using Fourier transform infrared spectroscopy, Brunauer–Emmett–Teller surface area analysis, thermogravimetric analysis, and transmission electron microscopy. We confirmed the formation of a nano-imprinted shell layer on the surface of Fe3O4@SiO2. The adsorption rate was fast, conforming to a pseudo-second-order kinetic and Langmuir adsorption model; the adsorption mechanism was deemed to be chemisorption as a single molecular layer. The maximum adsorption capacity of the IIP (51.2 mg g−1) was approximately three times as large as that of the non-imprinted polymer (17.9 mg g−1). The selectivity factors for Pb(II) in mixed solutions of Pb(II)/Co(II), Pb(II)/Cu(II), and Pb(II)/Zn(II) were 45.6, 6.45, and 8.3, respectively. Pb-IIP exhibited a high selectivity towards Pb(II), which enabled the enrichment of Pb(II) in aqueous solution.
中文翻译:

在磁铁矿纳米颗粒上轻松制备纳米压印聚合物,用于从水溶液中快速分离铅离子†
合成了一种新型的纳米结构磁性离子印迹聚合物(IIP),用于从水溶液中选择性吸附Pb(II)。IIP是在功能性Fe 3 O 4 @SiO 2核/壳纳米颗粒上作为载体制备的。聚合物中的单体单元具有典型的双齿配体衣康酸。我们分别使用乙二醇二甲基丙烯酸酯和2,2-偶氮异双丁腈作为交联剂和引发剂。研究了具有不同酸碱性质和不同比例的交联剂的单体,以获得高性能的吸附剂。我们的结果表明,从IIP衣康酸制备了由于强的单体和Pb之间的结合具有高吸附容量(II)模板离子。通过傅立叶变换红外光谱,Brunauer-Emmett-Teller表面积分析,热重分析和透射电子显微镜对IIP进行了表征。我们确认了在Fe 3 O 4 @SiO 2表面上形成了纳米压印壳层。吸附速度快,符合拟二级动力学和朗缪尔吸附模型;吸附机理被认为是化学吸附的单分子层。IIP(51.2 mg g -1)的最大吸附容量约为非压印聚合物(17.9 mg g -1)的最大吸附容量的三倍。铅(Pb )混合溶液中铅(II)的选择性因子II)/ Co(II),Pb(II)/ Cu(II)和Pb(II)/ Zn(II)分别为45.6、6.45和8.3。Pb-IIP对Pb(II)表现出高选择性,这使得Pb(II)在水溶液中富集。
更新日期:2018-04-19
中文翻译:

在磁铁矿纳米颗粒上轻松制备纳米压印聚合物,用于从水溶液中快速分离铅离子†
合成了一种新型的纳米结构磁性离子印迹聚合物(IIP),用于从水溶液中选择性吸附Pb(II)。IIP是在功能性Fe 3 O 4 @SiO 2核/壳纳米颗粒上作为载体制备的。聚合物中的单体单元具有典型的双齿配体衣康酸。我们分别使用乙二醇二甲基丙烯酸酯和2,2-偶氮异双丁腈作为交联剂和引发剂。研究了具有不同酸碱性质和不同比例的交联剂的单体,以获得高性能的吸附剂。我们的结果表明,从IIP衣康酸制备了由于强的单体和Pb之间的结合具有高吸附容量(II)模板离子。通过傅立叶变换红外光谱,Brunauer-Emmett-Teller表面积分析,热重分析和透射电子显微镜对IIP进行了表征。我们确认了在Fe 3 O 4 @SiO 2表面上形成了纳米压印壳层。吸附速度快,符合拟二级动力学和朗缪尔吸附模型;吸附机理被认为是化学吸附的单分子层。IIP(51.2 mg g -1)的最大吸附容量约为非压印聚合物(17.9 mg g -1)的最大吸附容量的三倍。铅(Pb )混合溶液中铅(II)的选择性因子II)/ Co(II),Pb(II)/ Cu(II)和Pb(II)/ Zn(II)分别为45.6、6.45和8.3。Pb-IIP对Pb(II)表现出高选择性,这使得Pb(II)在水溶液中富集。



























 京公网安备 11010802027423号
京公网安备 11010802027423号