Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of propellanes containing a bicyclo[2.2.2]octene unit via the Diels–Alder reaction and ring-closing metathesis as key steps†
RSC Advances ( IF 3.9 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1039/c8ra02687d Sambasivarao Kotha 1 , Sunil Pulletikurti 1
RSC Advances ( IF 3.9 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1039/c8ra02687d Sambasivarao Kotha 1 , Sunil Pulletikurti 1
Affiliation
|
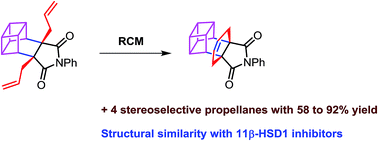
The synthesis of propellanes containing bicyclo[2.2.2]octene via olefin metathesis approach is less explored. Herein, we describe a simple and convenient method to synthesize propellane derivatives containing a bicyclo[2.2.2]octene unit which are structurally similar to 11β-HSD1 inhibitors by sequential usage of the Diels–Alder reaction, C-allylation and ring-closing metathesis (RCM) as the key steps. Additionally, we expanded this approach to an endo-tricyclo[4.2.2.02,5]decene derivative which is a useful monomer for polymer synthesis and we have also synthesized basketene and anthracene-based propellanes using the same strategy.
中文翻译:

通过 Diels-Alder 反应和闭环复分解作为关键步骤合成含有双环 [2.2.2] 辛烯单元的螺旋桨†
通过烯烃复分解方法合成含有双环[2.2.2]辛烯的螺旋桨的探索较少。在此,我们描述了一种简单方便的方法,通过依次使用 Diels-Alder 反应、C-烯丙基化和闭环复分解,合成含有双环 [2.2.2] 辛烯单元的螺旋烷衍生物,其结构类似于 11β-HSD1 抑制剂。 (RCM) 作为关键步骤。此外,我们将这种方法扩展到内-三环[4.2.2.0 2,5 ]癸烯衍生物,它是聚合物合成的有用单体,我们还使用相同的策略合成了基于篮子和蒽的螺旋桨。
更新日期:2018-04-19
中文翻译:

通过 Diels-Alder 反应和闭环复分解作为关键步骤合成含有双环 [2.2.2] 辛烯单元的螺旋桨†
通过烯烃复分解方法合成含有双环[2.2.2]辛烯的螺旋桨的探索较少。在此,我们描述了一种简单方便的方法,通过依次使用 Diels-Alder 反应、C-烯丙基化和闭环复分解,合成含有双环 [2.2.2] 辛烯单元的螺旋烷衍生物,其结构类似于 11β-HSD1 抑制剂。 (RCM) 作为关键步骤。此外,我们将这种方法扩展到内-三环[4.2.2.0 2,5 ]癸烯衍生物,它是聚合物合成的有用单体,我们还使用相同的策略合成了基于篮子和蒽的螺旋桨。











































 京公网安备 11010802027423号
京公网安备 11010802027423号