当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Absolute Configuration and Pharmacology of the Poison Frog Alkaloid Phantasmidine
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jnatprod.8b00062 Richard W. Fitch 1 , Barry B. Snider 2 , Quan Zhou 2 , Bruce M. Foxman 2 , Anshul A. Pandya 3 , Jerrel L. Yakel 3 , Thao T. Olson 4 , Nour Al-Muhtasib 4 , Yingxian Xiao 4 , Kevin D. Welch 5 , Kip E. Panter 5
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-04-19 00:00:00 , DOI: 10.1021/acs.jnatprod.8b00062 Richard W. Fitch 1 , Barry B. Snider 2 , Quan Zhou 2 , Bruce M. Foxman 2 , Anshul A. Pandya 3 , Jerrel L. Yakel 3 , Thao T. Olson 4 , Nour Al-Muhtasib 4 , Yingxian Xiao 4 , Kevin D. Welch 5 , Kip E. Panter 5
Affiliation
|
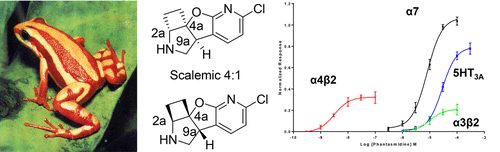
Phantasmidine, a rigid congener of the well-known nicotinic acetylcholine receptor agonist epibatidine, is found in the same species of poison frog (Epipedobates anthonyi). Natural phantasmidine was found to be a 4:1 scalemic mixture, enriched in the (2aR,4aS,9aS) enantiomer by chiral-phase LC-MS comparison to the synthetic enantiomers whose absolute configurations were previously established by Mosher’s amide analysis. The major enantiomer has the opposite S configuration at the benzylic carbon to natural epibatidine, whose benzylic carbon is R. Pharmacological characterization of the synthetic racemate and separated enantiomers established that phantasmidine is ∼10-fold less potent than epibatidine, but ∼100-fold more potent than nicotine in most receptors tested. Unlike epibatidine, phantasmidine is sharply enantioselective in its activity and the major natural enantiomer whose benzylic carbon has the 4aS configuration is more active. The stereoselective pharmacology of phantasmidine is ascribed to its rigid and asymmetric shape as compared to the nearly symmetric conformations previously suggested for epibatidine enantiomers. While phantasmidine itself is too toxic for direct therapeutic use, we believe it is a useful platform for the development of potent and selective nicotinic agonists, which may have value as pharmacological tools.
中文翻译:

毒蛙生物碱Ph的绝对构型和药理作用
在相同种类的毒蛙(Epipedobates anthonyi)中发现了Phantasmidine,它是众所周知的烟碱型乙酰胆碱受体激动剂Epibatidine的刚性同类物。通过手性液相色谱-质谱法与天然对映异构体进行比较,发现天然的潘他ta啶是一种4:1的比例混合物,富含(2a R,4a S,9a S)对映异构体,而合成对映异构体的绝对构型先前是通过Mosher酰胺分析确定的。主要对映异构体在苄基碳上具有与天然表巴替丁相反的S构型,后者的苄基碳为R。合成外消旋体和分离的对映异构体的药理学特征表明,在大多数测试的受体中,比他命啶的效价比表巴替丁低约10倍,但比尼古丁强约100倍。与Epibatidine不同,Pantatasmidine在活性上具有很强的对映选择性,而苄基碳原子具有4a S构型的主要天然对映体则更具活性。与以前对于Epibatidine对映异构体建议的几乎对称构象相比,phantasmidine的立体选择性药理学归因于其刚性和不对称的形状。虽然phantasmidine本身毒性太大,无法直接用于治疗,但我们认为它是开发有效和选择性烟碱激动剂的有用平台,这些激动剂可能具有药理作用。
更新日期:2018-04-19
中文翻译:

毒蛙生物碱Ph的绝对构型和药理作用
在相同种类的毒蛙(Epipedobates anthonyi)中发现了Phantasmidine,它是众所周知的烟碱型乙酰胆碱受体激动剂Epibatidine的刚性同类物。通过手性液相色谱-质谱法与天然对映异构体进行比较,发现天然的潘他ta啶是一种4:1的比例混合物,富含(2a R,4a S,9a S)对映异构体,而合成对映异构体的绝对构型先前是通过Mosher酰胺分析确定的。主要对映异构体在苄基碳上具有与天然表巴替丁相反的S构型,后者的苄基碳为R。合成外消旋体和分离的对映异构体的药理学特征表明,在大多数测试的受体中,比他命啶的效价比表巴替丁低约10倍,但比尼古丁强约100倍。与Epibatidine不同,Pantatasmidine在活性上具有很强的对映选择性,而苄基碳原子具有4a S构型的主要天然对映体则更具活性。与以前对于Epibatidine对映异构体建议的几乎对称构象相比,phantasmidine的立体选择性药理学归因于其刚性和不对称的形状。虽然phantasmidine本身毒性太大,无法直接用于治疗,但我们认为它是开发有效和选择性烟碱激动剂的有用平台,这些激动剂可能具有药理作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号