Chem ( IF 19.1 ) Pub Date : 2018-04-19 , DOI: 10.1016/j.chempr.2018.03.008 Jun Pan , Xinyao Li , Fengguirong Lin , Jianzhong Liu , Ning Jiao
|
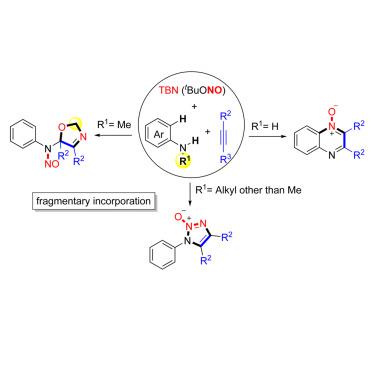
The cycloaddition reactions have been explored extensively and provided an efficient strategy for the synthesis of cyclic compounds. Traditionally, the reaction partners were in extenso incorporated into the cyclic products without fragmentation. From a different perspective, if certain fragmentations via chemical-bond cleavage are involved in this cycloaddition reaction, it would change the assembly sequence and enable more product diversity. Here, we report a chemoselective nitrosylation of anilines and alkynes through fragmentary or complete NO radical incorporation. The formation of multiple C–N bonds, an unexpected C–N bond, and N=O bond cleavage make this fragmentary cycloaddition reaction an efficient approach to 2,5-dihydrooxazoles, 1H-1,2,3-triazole 2-oxides or quinoxaline N-oxides. Facile operation in open-air, metal-free, and mild conditions renders this protocol particularly practical and attractive. A series of mechanistic studies and density functional theory calculations were also conducted, which help to explain the fragmentary or complete NO incorporation processes, broadening the field of new reaction discovery.
中文翻译:

苯胺和炔烃通过部分或完全NO引入的化学选择性硝基化
环加成反应已被广泛研究,并为合成环状化合物提供了有效的策略。传统上,反应伙伴被广泛地掺入环状产物中而不会断裂。从不同的角度来看,如果在这种环加成反应中涉及通过化学键裂解的某些片段化,它将改变组装顺序并实现更多的产物多样性。在这里,我们报告通过部分或完全的NO自由基结合苯胺和炔烃的化学选择性亚硝基化。多个C–N键,意外的C–N键和N = O键断裂的形成使该片段性环加成反应成为2,5,2-二氢恶唑,1 H -1,2,3-三唑2-氧化物的有效方法或喹喔啉N-氧化物。在露天,无金属和温和条件下的简便操作使该协议特别实用且有吸引力。还进行了一系列的机理研究和密度泛函理论计算,这有助于解释零碎或完整的NO掺入过程,拓宽了新反应发现的领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号