当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of O-GlcNAc transferase (OGT) by peptidic hybrids†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1039/c8md00115d Hao Zhang 1 , Tihomir Tomašič 2 , Jie Shi 1 , Matjaž Weiss 2 , Rob Ruijtenbeek 1, 3 , Marko Anderluh 2 , Roland J Pieters 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1039/c8md00115d Hao Zhang 1 , Tihomir Tomašič 2 , Jie Shi 1 , Matjaž Weiss 2 , Rob Ruijtenbeek 1, 3 , Marko Anderluh 2 , Roland J Pieters 1
Affiliation
|
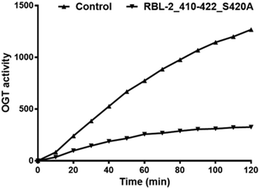
O-GlcNAc transferase (OGT) attaches a GlcNAc moiety on specific substrate proteins using UDP-GlcNAc as the sugar donor. This modification can alter protein function by regulating cellular signaling and transcription pathways in response to altered nutrient availability and stress. Specific inhibitors of OGT would be valuable tools for biological studies and lead structures for therapeutics. The existing OGT inhibitors are mainly derived from the sugar donor substrate, but poor cell permeability and off-target effects limit their use. Here, we describe our progress on OGT inhibition based on substrate peptides identified by array screening. Subsequently, bisubstrate inhibitors were prepared by conjugating these peptides to uridine in various ways. In parallel, an in silico fragment screening was conducted to obtain small molecules targeting the UDP binding pocket. After evaluation of the initial hits, one of these small molecules was elaborated into a novel OGT hybrid inhibitor, as the replacement of uridine. The novel compounds inhibit OGT activity with IC50 values in the micromolar range.
中文翻译:

肽杂合体抑制 O-GlcNAc 转移酶 (OGT)†
O -GlcNAc 转移酶 (OGT) 使用 UDP-GlcNAc 作为糖供体将 GlcNAc 部分附着在特定底物蛋白上。这种修饰可以通过调节细胞信号传导和转录途径来改变蛋白质功能,以响应营养可用性和压力的变化。 OGT 的特异性抑制剂将成为生物学研究的宝贵工具和治疗学的先导结构。现有的OGT抑制剂主要来源于糖供体底物,但细胞通透性差和脱靶效应限制了其使用。在这里,我们描述了基于阵列筛选鉴定的底物肽在 OGT 抑制方面的进展。随后,通过以各种方式将这些肽与尿苷缀合来制备双底物抑制剂。同时,进行了计算机片段筛选以获得靶向 UDP 结合口袋的小分子。在对最初的命中进行评估后,其中一个小分子被精心制作成一种新型 OGT 混合抑制剂,作为尿苷的替代品。这些新型化合物抑制 OGT 活性,IC 50值在微摩尔范围内。
更新日期:2018-04-18
中文翻译:

肽杂合体抑制 O-GlcNAc 转移酶 (OGT)†
O -GlcNAc 转移酶 (OGT) 使用 UDP-GlcNAc 作为糖供体将 GlcNAc 部分附着在特定底物蛋白上。这种修饰可以通过调节细胞信号传导和转录途径来改变蛋白质功能,以响应营养可用性和压力的变化。 OGT 的特异性抑制剂将成为生物学研究的宝贵工具和治疗学的先导结构。现有的OGT抑制剂主要来源于糖供体底物,但细胞通透性差和脱靶效应限制了其使用。在这里,我们描述了基于阵列筛选鉴定的底物肽在 OGT 抑制方面的进展。随后,通过以各种方式将这些肽与尿苷缀合来制备双底物抑制剂。同时,进行了计算机片段筛选以获得靶向 UDP 结合口袋的小分子。在对最初的命中进行评估后,其中一个小分子被精心制作成一种新型 OGT 混合抑制剂,作为尿苷的替代品。这些新型化合物抑制 OGT 活性,IC 50值在微摩尔范围内。









































 京公网安备 11010802027423号
京公网安备 11010802027423号