当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of fused pyrroles containing 4-hydroxycoumarins by regioselective metal-free multicomponent reactions†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1039/c8ob00161h Richa Mishra 1, 2, 3, 4 , Asim Jana 1, 2, 3, 4 , Anoop Kumar Panday 1, 2, 3, 4 , Lokman H. Choudhury 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1039/c8ob00161h Richa Mishra 1, 2, 3, 4 , Asim Jana 1, 2, 3, 4 , Anoop Kumar Panday 1, 2, 3, 4 , Lokman H. Choudhury 1, 2, 3, 4
Affiliation
|
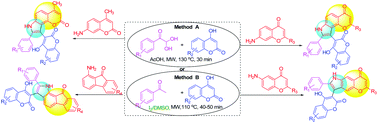
The reaction of arylglyoxals, 4-hydroxycoumarin, and aromatic amines such as 7-amino-2-methylchromone, 6/7-aminoflavone, 7-amino-4-methylcoumarin, 1-amino-9-fluorenone, 1-aminoanthraquinone and aniline derivatives in acetic acid medium under microwave conditions provides the corresponding regioselective fused pyrroles having hydroxycoumarin and aryl substituents. Alternatively, we have developed another method using in situ arylglyoxals from acetophenone derivatives by I2/DMSO promoted C–H oxidation followed by one-pot three component cyclization reactions to provide similar fused pyrroles. Using both the methods a series of novel pyrroles fused with pharmacologically important chromone, flavone, coumarin, fluorenone, and anthraquinone moieties were synthesized under metal-free reaction conditions in good to very good yields within a short reaction time. The structures of the synthesized fused pyrroles have been unambiguously confirmed by spectroscopic techniques, mass analysis and single crystal XRD.
中文翻译:

通过区域选择性无金属多组分反应合成包含4-羟基香豆素的稠合吡咯†
芳基乙二醛,4-羟基香豆素和芳香胺如7-氨基-2-甲基色酮,6 / 7-氨基黄酮,7-氨基-4-甲基香豆素,1-氨基-9-芴酮,1-氨基蒽醌和苯胺衍生物的反应在微波条件下,在乙酸介质中,提供相应的具有羟基香豆素和芳基取代基的区域选择性稠合的吡咯。或者,我们已经开发了另一种方法,该方法利用苯乙酮衍生物的原位芳基乙二醛通过I 2/ DMSO促进C–H氧化,然后进行一锅三组分环化反应以提供相似的稠合吡咯。使用这两种方法,在无金属反应条件下,在短时间内,以良好或非常好的收率合成了一系列新颖的吡咯,它们与具有重要药理作用的色酮,黄酮,香豆素,芴酮和蒽醌部分融合。合成的稠合吡咯的结构已通过光谱技术,质量分析和单晶XRD明确确认。
更新日期:2018-04-18
中文翻译:

通过区域选择性无金属多组分反应合成包含4-羟基香豆素的稠合吡咯†
芳基乙二醛,4-羟基香豆素和芳香胺如7-氨基-2-甲基色酮,6 / 7-氨基黄酮,7-氨基-4-甲基香豆素,1-氨基-9-芴酮,1-氨基蒽醌和苯胺衍生物的反应在微波条件下,在乙酸介质中,提供相应的具有羟基香豆素和芳基取代基的区域选择性稠合的吡咯。或者,我们已经开发了另一种方法,该方法利用苯乙酮衍生物的原位芳基乙二醛通过I 2/ DMSO促进C–H氧化,然后进行一锅三组分环化反应以提供相似的稠合吡咯。使用这两种方法,在无金属反应条件下,在短时间内,以良好或非常好的收率合成了一系列新颖的吡咯,它们与具有重要药理作用的色酮,黄酮,香豆素,芴酮和蒽醌部分融合。合成的稠合吡咯的结构已通过光谱技术,质量分析和单晶XRD明确确认。











































 京公网安备 11010802027423号
京公网安备 11010802027423号