当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Denitrogenative palladium-catalyzed coupling of aryl halides with arylhydrazines under mild conditions†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1039/c8ob00305j Sheng Chang 1, 2, 3, 4 , Lin Lin Dong 1, 3, 4, 5 , Hai Qing Song 1, 2, 3, 4 , Bo Feng 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1039/c8ob00305j Sheng Chang 1, 2, 3, 4 , Lin Lin Dong 1, 3, 4, 5 , Hai Qing Song 1, 2, 3, 4 , Bo Feng 1, 2, 3, 4
Affiliation
|
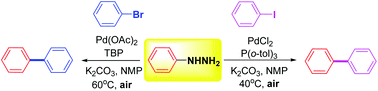
The development of a method for the Pd(II)-catalyzed denitrogenative coupling of arylhydrazines to give functionalized biaryls in good yield, using aryl bromides or aryl iodides as convenient and inexpensive aryl sources, is reported. High functional group tolerance is demonstrated for electronically distinct arylhydrazines as well as aryl halides. The desired products were isolated in good to excellent yields for 58 examples. Control experiments and mechanism studies revealed that the transformation undergoes a base-promoted Pd-catalyzed process.
中文翻译:

在温和条件下钯卤代芳基卤化物与芳基肼的脱氮钯催化偶联†
据报道,使用芳基溴化物或芳基碘化物作为方便且廉价的芳基来源,开发了用于Pd(II)催化的芳基肼的脱氮偶联以高收率得到官能化联芳基的方法。对于电子上不同的芳基肼以及芳基卤化物,证明了较高的官能团耐受性。对于58个实施例,以良好至优异的产率分离出所需产物。对照实验和机理研究表明,转化经历了碱促进的Pd催化过程。
更新日期:2018-04-18
中文翻译:

在温和条件下钯卤代芳基卤化物与芳基肼的脱氮钯催化偶联†
据报道,使用芳基溴化物或芳基碘化物作为方便且廉价的芳基来源,开发了用于Pd(II)催化的芳基肼的脱氮偶联以高收率得到官能化联芳基的方法。对于电子上不同的芳基肼以及芳基卤化物,证明了较高的官能团耐受性。对于58个实施例,以良好至优异的产率分离出所需产物。对照实验和机理研究表明,转化经历了碱促进的Pd催化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号