Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, characterization and sorption studies of aromatic compounds by hydrogels of chitosan blended with β-cyclodextrin- and PVA-functionalized pectin†
RSC Advances ( IF 3.9 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1039/c8ra02332h Cesar M C Filho 1 , Pedro V A Bueno 2 , Alan F Y Matsushita 1 , Adley F Rubira 2 , Edvani C Muniz 2, 3 , Luísa Durães 4 , Dina M B Murtinho 1 , Artur J M Valente 1
RSC Advances ( IF 3.9 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1039/c8ra02332h Cesar M C Filho 1 , Pedro V A Bueno 2 , Alan F Y Matsushita 1 , Adley F Rubira 2 , Edvani C Muniz 2, 3 , Luísa Durães 4 , Dina M B Murtinho 1 , Artur J M Valente 1
Affiliation
|
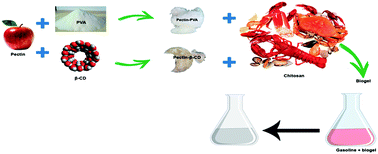
Petroleum comprises the monoaromatic and polycyclic aromatic hydrocarbons, which exhibit acute toxicity towards living animals. Consequently, their removal from natural environment is a priority challenge. On the other hand, biomaterials are increasingly being used as adsorbents. Pectin and chitosan are well-known polysaccharides able to form coacervate hydrogels. Aiming an increase of sorption ability by hydrophobic compounds, pectin was also functionalized with two amphiphilic compounds: β-cyclodextrin (β-CD) and poly(vinyl alcohol) (PVA). Both the modified pectin and the hydrogels were evaluated using nuclear magnetic resonance (NMR), infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). The hydrogels were further characterized in terms of thermogravimetric analysis (TGA) and swelling kinetics. The interaction between the hydrogel and mix solutions containing six different aromatic compounds (BTXs and the following PAHs: pyrene, benzo(b)fluoranthene and benzo(a)pyrene) has been evaluated through sorption isotherms and kinetics. The mechanism of sorption interaction and the selectivity of the adsorbents towards different aromatic compounds were discussed. The results clearly show that the presence of β-CD and PVA into gel leads to an increase in the removal efficiency of both, BTXs and PAHs. The gels were subjected to two sorption/desorption cycles to have an assessment of the capability of adsorbents for re-use. Finally, the sorption quantification of those six aromatic compounds from a real gasoline sample onto gels has been tested.
中文翻译:

壳聚糖与 β-环糊精和 PVA 功能化果胶混合的水凝胶对芳香族化合物的合成、表征和吸附研究†
石油包含单芳烃和多环芳烃,它们对活体动物表现出急性毒性。因此,将它们从自然环境中清除是一项优先挑战。另一方面,生物材料越来越多地被用作吸附剂。果胶和壳聚糖是众所周知的能够形成凝聚水凝胶的多糖。为了提高疏水性化合物的吸附能力,果胶还用两种两亲性化合物进行了功能化:β-环糊精 (β-CD) 和聚乙烯醇 (PVA)。使用核磁共振 (NMR)、红外光谱 (FTIR) 和扫描电子显微镜 (SEM) 对改性果胶和水凝胶进行了评估。在热重分析(TGA)和溶胀动力学方面进一步表征了水凝胶。已通过吸附等温线和动力学评估了水凝胶和含有六种不同芳族化合物(BTX 和以下 PAH:芘、苯并 (b) 荧蒽和苯并 (a) 芘)的混合溶液之间的相互作用。讨论了吸附相互作用的机理和吸附剂对不同芳香族化合物的选择性。结果清楚地表明,凝胶中 β-CD 和 PVA 的存在导致 BTX 和 PAH 的去除效率增加。对凝胶进行两次吸附/解吸循环以评估吸附剂重复使用的能力。最后,测试了真实汽油样品中这六种芳香族化合物在凝胶上的吸附定量。苯并(b)荧蒽和苯并(a)芘)已通过吸附等温线和动力学进行了评估。讨论了吸附相互作用的机理和吸附剂对不同芳香族化合物的选择性。结果清楚地表明,凝胶中 β-CD 和 PVA 的存在导致 BTX 和 PAH 的去除效率增加。对凝胶进行两次吸附/解吸循环以评估吸附剂重复使用的能力。最后,测试了真实汽油样品中这六种芳香族化合物在凝胶上的吸附定量。苯并(b)荧蒽和苯并(a)芘)已通过吸附等温线和动力学进行了评估。讨论了吸附相互作用的机理和吸附剂对不同芳香族化合物的选择性。结果清楚地表明,凝胶中 β-CD 和 PVA 的存在导致 BTX 和 PAH 的去除效率增加。对凝胶进行两次吸附/解吸循环以评估吸附剂重复使用的能力。最后,测试了真实汽油样品中这六种芳香族化合物在凝胶上的吸附定量。结果清楚地表明,凝胶中 β-CD 和 PVA 的存在导致 BTX 和 PAH 的去除效率增加。对凝胶进行两次吸附/解吸循环以评估吸附剂重复使用的能力。最后,测试了真实汽油样品中这六种芳香族化合物在凝胶上的吸附定量。结果清楚地表明,凝胶中 β-CD 和 PVA 的存在导致 BTX 和 PAH 的去除效率增加。对凝胶进行两次吸附/解吸循环以评估吸附剂重复使用的能力。最后,测试了真实汽油样品中这六种芳香族化合物在凝胶上的吸附定量。
更新日期:2018-04-18
中文翻译:

壳聚糖与 β-环糊精和 PVA 功能化果胶混合的水凝胶对芳香族化合物的合成、表征和吸附研究†
石油包含单芳烃和多环芳烃,它们对活体动物表现出急性毒性。因此,将它们从自然环境中清除是一项优先挑战。另一方面,生物材料越来越多地被用作吸附剂。果胶和壳聚糖是众所周知的能够形成凝聚水凝胶的多糖。为了提高疏水性化合物的吸附能力,果胶还用两种两亲性化合物进行了功能化:β-环糊精 (β-CD) 和聚乙烯醇 (PVA)。使用核磁共振 (NMR)、红外光谱 (FTIR) 和扫描电子显微镜 (SEM) 对改性果胶和水凝胶进行了评估。在热重分析(TGA)和溶胀动力学方面进一步表征了水凝胶。已通过吸附等温线和动力学评估了水凝胶和含有六种不同芳族化合物(BTX 和以下 PAH:芘、苯并 (b) 荧蒽和苯并 (a) 芘)的混合溶液之间的相互作用。讨论了吸附相互作用的机理和吸附剂对不同芳香族化合物的选择性。结果清楚地表明,凝胶中 β-CD 和 PVA 的存在导致 BTX 和 PAH 的去除效率增加。对凝胶进行两次吸附/解吸循环以评估吸附剂重复使用的能力。最后,测试了真实汽油样品中这六种芳香族化合物在凝胶上的吸附定量。苯并(b)荧蒽和苯并(a)芘)已通过吸附等温线和动力学进行了评估。讨论了吸附相互作用的机理和吸附剂对不同芳香族化合物的选择性。结果清楚地表明,凝胶中 β-CD 和 PVA 的存在导致 BTX 和 PAH 的去除效率增加。对凝胶进行两次吸附/解吸循环以评估吸附剂重复使用的能力。最后,测试了真实汽油样品中这六种芳香族化合物在凝胶上的吸附定量。苯并(b)荧蒽和苯并(a)芘)已通过吸附等温线和动力学进行了评估。讨论了吸附相互作用的机理和吸附剂对不同芳香族化合物的选择性。结果清楚地表明,凝胶中 β-CD 和 PVA 的存在导致 BTX 和 PAH 的去除效率增加。对凝胶进行两次吸附/解吸循环以评估吸附剂重复使用的能力。最后,测试了真实汽油样品中这六种芳香族化合物在凝胶上的吸附定量。结果清楚地表明,凝胶中 β-CD 和 PVA 的存在导致 BTX 和 PAH 的去除效率增加。对凝胶进行两次吸附/解吸循环以评估吸附剂重复使用的能力。最后,测试了真实汽油样品中这六种芳香族化合物在凝胶上的吸附定量。结果清楚地表明,凝胶中 β-CD 和 PVA 的存在导致 BTX 和 PAH 的去除效率增加。对凝胶进行两次吸附/解吸循环以评估吸附剂重复使用的能力。最后,测试了真实汽油样品中这六种芳香族化合物在凝胶上的吸附定量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号