Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis of ligand binding modes at the neuropeptide Y Y1 receptor
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0046-x Zhenlin Yang 1, 2 , Shuo Han 1, 3 , Max Keller 4 , Anette Kaiser 5 , Brian J Bender 6 , Mathias Bosse 7 , Kerstin Burkert 5 , Lisa M Kögler 5 , David Wifling 4 , Guenther Bernhardt 4 , Nicole Plank 4 , Timo Littmann 4 , Peter Schmidt 7 , Cuiying Yi 1 , Beibei Li 1, 8 , Sheng Ye 3 , Rongguang Zhang 3, 9 , Bo Xu 10 , Dan Larhammar 10 , Raymond C Stevens 11, 12 , Daniel Huster 7 , Jens Meiler 6, 13 , Qiang Zhao 1, 2, 8, 14 , Annette G Beck-Sickinger 5 , Armin Buschauer 4 , Beili Wu 1, 8, 12, 14
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0046-x Zhenlin Yang 1, 2 , Shuo Han 1, 3 , Max Keller 4 , Anette Kaiser 5 , Brian J Bender 6 , Mathias Bosse 7 , Kerstin Burkert 5 , Lisa M Kögler 5 , David Wifling 4 , Guenther Bernhardt 4 , Nicole Plank 4 , Timo Littmann 4 , Peter Schmidt 7 , Cuiying Yi 1 , Beibei Li 1, 8 , Sheng Ye 3 , Rongguang Zhang 3, 9 , Bo Xu 10 , Dan Larhammar 10 , Raymond C Stevens 11, 12 , Daniel Huster 7 , Jens Meiler 6, 13 , Qiang Zhao 1, 2, 8, 14 , Annette G Beck-Sickinger 5 , Armin Buschauer 4 , Beili Wu 1, 8, 12, 14
Affiliation
|
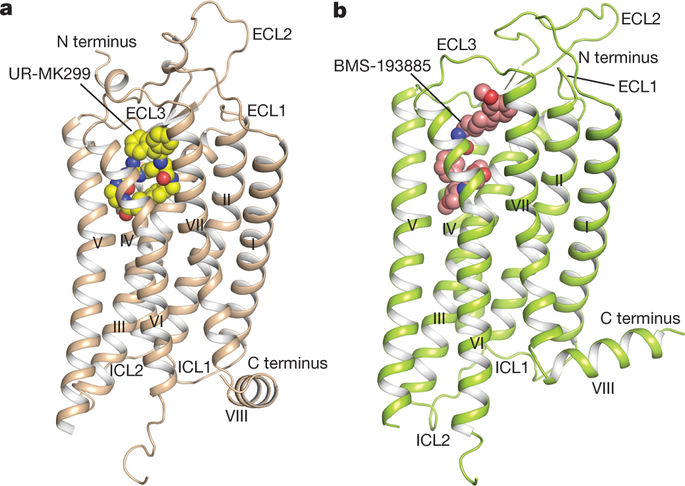
Neuropeptide Y (NPY) receptors belong to the G-protein-coupled receptor superfamily and have important roles in food intake, anxiety and cancer biology1,2. The NPY–Y receptor system has emerged as one of the most complex networks with three peptide ligands (NPY, peptide YY and pancreatic polypeptide) binding to four receptors in most mammals, namely the Y1, Y2, Y4 and Y5 receptors, with different affinity and selectivity3. NPY is the most powerful stimulant of food intake and this effect is primarily mediated by the Y1 receptor (Y1R)4. A number of peptides and small-molecule compounds have been characterized as Y1R antagonists and have shown clinical potential in the treatment of obesity4, tumour1 and bone loss5. However, their clinical usage has been hampered by low potency and selectivity, poor brain penetration ability or lack of oral bioavailability6. Here we report crystal structures of the human Y1R bound to the two selective antagonists UR-MK299 and BMS-193885 at 2.7 and 3.0 Å resolution, respectively. The structures combined with mutagenesis studies reveal the binding modes of Y1R to several structurally diverse antagonists and the determinants of ligand selectivity. The Y1R structure and molecular docking of the endogenous agonist NPY, together with nuclear magnetic resonance, photo-crosslinking and functional studies, provide insights into the binding behaviour of the agonist and for the first time, to our knowledge, determine the interaction of its N terminus with the receptor. These insights into Y1R can enable structure-based drug discovery that targets NPY receptors.Crystal structures of the neuropeptide Y1 receptor in complex with two distinct antagonists combined with NMR, molecular docking and mutagenesis studies inform a proposed model for receptor–agonist binding.
中文翻译:

神经肽 Y Y1 受体配体结合模式的结构基础
神经肽 Y (NPY) 受体属于 G 蛋白偶联受体超家族,在食物摄入、焦虑和癌症生物学中具有重要作用 1,2。NPY-Y 受体系统已成为最复杂的网络之一,具有三种肽配体(NPY、肽 YY 和胰多肽)与大多数哺乳动物中的四种受体(即 Y1、Y2、Y4 和 Y5 受体)结合,具有不同的亲和力和选择性3。NPY 是最强大的食物摄入兴奋剂,这种作用主要由 Y1 受体 (Y1R)4 介导。许多肽和小分子化合物已被表征为 Y1R 拮抗剂,并已显示出治疗肥胖 4、肿瘤 1 和骨质流失 5 的临床潜力。然而,它们的临床应用受到低效力和选择性的阻碍,脑渗透能力差或缺乏口服生物利用度6。在这里,我们分别报告了与两种选择性拮抗剂 UR-MK299 和 BMS-193885 结合的人类 Y1R 的晶体结构,分辨率分别为 2.7 和 3.0 Å。结合诱变研究的结构揭示了 Y1R 与几种结构不同的拮抗剂的结合模式和配体选择性的决定因素。内源性激动剂 NPY 的 Y1R 结构和分子对接,连同核磁共振、光交联和功能研究,提供了对激动剂结合行为的深入了解,据我们所知,这是第一次确定其 N 的相互作用末端与受体。这些对 Y1R 的见解可以实现针对 NPY 受体的基于结构的药物发现。
更新日期:2018-04-01
中文翻译:

神经肽 Y Y1 受体配体结合模式的结构基础
神经肽 Y (NPY) 受体属于 G 蛋白偶联受体超家族,在食物摄入、焦虑和癌症生物学中具有重要作用 1,2。NPY-Y 受体系统已成为最复杂的网络之一,具有三种肽配体(NPY、肽 YY 和胰多肽)与大多数哺乳动物中的四种受体(即 Y1、Y2、Y4 和 Y5 受体)结合,具有不同的亲和力和选择性3。NPY 是最强大的食物摄入兴奋剂,这种作用主要由 Y1 受体 (Y1R)4 介导。许多肽和小分子化合物已被表征为 Y1R 拮抗剂,并已显示出治疗肥胖 4、肿瘤 1 和骨质流失 5 的临床潜力。然而,它们的临床应用受到低效力和选择性的阻碍,脑渗透能力差或缺乏口服生物利用度6。在这里,我们分别报告了与两种选择性拮抗剂 UR-MK299 和 BMS-193885 结合的人类 Y1R 的晶体结构,分辨率分别为 2.7 和 3.0 Å。结合诱变研究的结构揭示了 Y1R 与几种结构不同的拮抗剂的结合模式和配体选择性的决定因素。内源性激动剂 NPY 的 Y1R 结构和分子对接,连同核磁共振、光交联和功能研究,提供了对激动剂结合行为的深入了解,据我们所知,这是第一次确定其 N 的相互作用末端与受体。这些对 Y1R 的见解可以实现针对 NPY 受体的基于结构的药物发现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号