Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Genetic identification of leptin neural circuits in energy and glucose homeostases
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0049-7 Jie Xu , Christopher L. Bartolome , Cho Shing Low , Xinchi Yi , Cheng-Hao Chien , Peng Wang , Dong Kong
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0049-7 Jie Xu , Christopher L. Bartolome , Cho Shing Low , Xinchi Yi , Cheng-Hao Chien , Peng Wang , Dong Kong
|
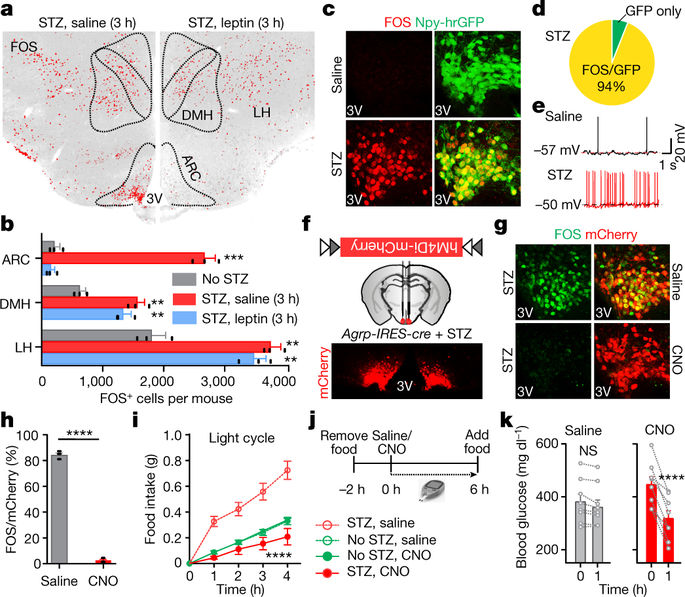
Leptin, a hormone produced in white adipose tissue, acts in the brain to communicate fuel status, suppress appetite following a meal, promote energy expenditure and maintain blood glucose stability1,2. Dysregulation of leptin or its receptors (LEPR) results in severe obesity and diabetes3–5. Although intensive studies on leptin have transformed obesity and diabetes research2,6, clinical applications of the molecule are still limited7, at least in part owing to the complexity and our incomplete understanding of the underlying neural circuits. The hypothalamic neurons that express agouti-related peptide (AGRP) and pro-opiomelanocortin (POMC) have been hypothesized to be the main first-order, leptin-responsive neurons. Selective deletion of LEPR in these neurons with the Cre–loxP system, however, has previously failed to recapitulate, or only marginally recapitulated, the obesity and diabetes that are seen in LEPR-deficient Leprdb/db mice, suggesting that AGRP or POMC neurons are not directly required for the effects of leptin in vivo8–10. The primary neural targets of leptin are therefore still unclear. Here we conduct a systematic, unbiased survey of leptin-responsive neurons in streptozotocin-induced diabetic mice and exploit CRISPR–Cas9-mediated genetic ablation of LEPR in vivo. Unexpectedly, we find that AGRP neurons but not POMC neurons are required for the primary action of leptin to regulate both energy balance and glucose homeostasis. Leptin deficiency disinhibits AGRP neurons, and chemogenetic inhibition of these neurons reverses both diabetic hyperphagia and hyperglycaemia. In sharp contrast to previous studies, we show that CRISPR-mediated deletion of LEPR in AGRP neurons causes severe obesity and diabetes, faithfully replicating the phenotype of Leprdb/db mice. We also uncover divergent mechanisms of acute and chronic inhibition of AGRP neurons by leptin (presynaptic potentiation of GABA (γ-aminobutyric acid) neurotransmission and postsynaptic activation of ATP-sensitive potassium channels, respectively). Our findings identify the underlying basis of the neurobiological effects of leptin and associated metabolic disorders.A subset of neurons in the hypothalamus is identified as the primary site of action for regulating energy balance and glucose homeostasis by leptin.
中文翻译:

能量和葡萄糖稳态中瘦素神经回路的遗传鉴定
瘦素是一种在白色脂肪组织中产生的激素,在大脑中起作用以传达燃料状态、抑制饭后食欲、促进能量消耗并维持血糖稳定 1,2。瘦素或其受体 (LEPR) 的失调会导致严重的肥胖症和糖尿病 3-5。尽管对瘦素的深入研究已经改变了肥胖和糖尿病的研究 2,6,但该分子的临床应用仍然有限 7,至少部分原因是由于其复杂性和我们对潜在神经回路的不完全了解。表达刺鼠相关肽 (AGRP) 和阿片黑素原 (POMC) 的下丘脑神经元被假设为主要的一级、瘦素反应性神经元。然而,使用 Cre-loxP 系统选择性删除这些神经元中的 LEPR,然而,以前未能重述,或者只是稍微概括一下,在 LEPR 缺陷的 Leprdb/db 小鼠中观察到的肥胖和糖尿病,表明 AGRP 或 POMC 神经元不是体内瘦素作用直接需要的 8-10。因此,瘦素的主要神经靶点仍不清楚。在这里,我们对链脲佐菌素诱导的糖尿病小鼠中的瘦素反应性神经元进行了系统的、公正的调查,并在体内利用 CRISPR-Cas9 介导的 LEPR 基因消融。出乎意料的是,我们发现瘦素调节能量平衡和葡萄糖稳态的主要作用需要AGRP神经元而不是POMC神经元。瘦素缺乏会抑制 AGRP 神经元,并且这些神经元的化学遗传学抑制可以逆转糖尿病性食欲亢进症和高血糖症。与以往的研究形成鲜明对比的是,我们表明,CRISPR 介导的 AGRP 神经元中 LEPR 的缺失会导致严重的肥胖和糖尿病,忠实地复制了 Leprdb/db 小鼠的表型。我们还揭示了瘦素(分别是 GABA(γ-氨基丁酸)神经传递的突触前增强和 ATP 敏感钾通道的突触后激活)对 AGRP 神经元的急性和慢性抑制的不同机制。我们的研究结果确定了瘦素和相关代谢紊乱的神经生物学效应的潜在基础。下丘脑中的一部分神经元被确定为瘦素调节能量平衡和葡萄糖稳态的主要作用部位。我们还揭示了瘦素(分别是 GABA(γ-氨基丁酸)神经传递的突触前增强和 ATP 敏感钾通道的突触后激活)对 AGRP 神经元的急性和慢性抑制的不同机制。我们的研究结果确定了瘦素和相关代谢紊乱的神经生物学效应的潜在基础。下丘脑中的一部分神经元被确定为瘦素调节能量平衡和葡萄糖稳态的主要作用部位。我们还揭示了瘦素(分别是 GABA(γ-氨基丁酸)神经传递的突触前增强和 ATP 敏感钾通道的突触后激活)对 AGRP 神经元的急性和慢性抑制的不同机制。我们的研究结果确定了瘦素和相关代谢紊乱的神经生物学效应的潜在基础。下丘脑中的一部分神经元被确定为瘦素调节能量平衡和葡萄糖稳态的主要作用部位。
更新日期:2018-04-01
中文翻译:

能量和葡萄糖稳态中瘦素神经回路的遗传鉴定
瘦素是一种在白色脂肪组织中产生的激素,在大脑中起作用以传达燃料状态、抑制饭后食欲、促进能量消耗并维持血糖稳定 1,2。瘦素或其受体 (LEPR) 的失调会导致严重的肥胖症和糖尿病 3-5。尽管对瘦素的深入研究已经改变了肥胖和糖尿病的研究 2,6,但该分子的临床应用仍然有限 7,至少部分原因是由于其复杂性和我们对潜在神经回路的不完全了解。表达刺鼠相关肽 (AGRP) 和阿片黑素原 (POMC) 的下丘脑神经元被假设为主要的一级、瘦素反应性神经元。然而,使用 Cre-loxP 系统选择性删除这些神经元中的 LEPR,然而,以前未能重述,或者只是稍微概括一下,在 LEPR 缺陷的 Leprdb/db 小鼠中观察到的肥胖和糖尿病,表明 AGRP 或 POMC 神经元不是体内瘦素作用直接需要的 8-10。因此,瘦素的主要神经靶点仍不清楚。在这里,我们对链脲佐菌素诱导的糖尿病小鼠中的瘦素反应性神经元进行了系统的、公正的调查,并在体内利用 CRISPR-Cas9 介导的 LEPR 基因消融。出乎意料的是,我们发现瘦素调节能量平衡和葡萄糖稳态的主要作用需要AGRP神经元而不是POMC神经元。瘦素缺乏会抑制 AGRP 神经元,并且这些神经元的化学遗传学抑制可以逆转糖尿病性食欲亢进症和高血糖症。与以往的研究形成鲜明对比的是,我们表明,CRISPR 介导的 AGRP 神经元中 LEPR 的缺失会导致严重的肥胖和糖尿病,忠实地复制了 Leprdb/db 小鼠的表型。我们还揭示了瘦素(分别是 GABA(γ-氨基丁酸)神经传递的突触前增强和 ATP 敏感钾通道的突触后激活)对 AGRP 神经元的急性和慢性抑制的不同机制。我们的研究结果确定了瘦素和相关代谢紊乱的神经生物学效应的潜在基础。下丘脑中的一部分神经元被确定为瘦素调节能量平衡和葡萄糖稳态的主要作用部位。我们还揭示了瘦素(分别是 GABA(γ-氨基丁酸)神经传递的突触前增强和 ATP 敏感钾通道的突触后激活)对 AGRP 神经元的急性和慢性抑制的不同机制。我们的研究结果确定了瘦素和相关代谢紊乱的神经生物学效应的潜在基础。下丘脑中的一部分神经元被确定为瘦素调节能量平衡和葡萄糖稳态的主要作用部位。我们还揭示了瘦素(分别是 GABA(γ-氨基丁酸)神经传递的突触前增强和 ATP 敏感钾通道的突触后激活)对 AGRP 神经元的急性和慢性抑制的不同机制。我们的研究结果确定了瘦素和相关代谢紊乱的神经生物学效应的潜在基础。下丘脑中的一部分神经元被确定为瘦素调节能量平衡和葡萄糖稳态的主要作用部位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号