当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
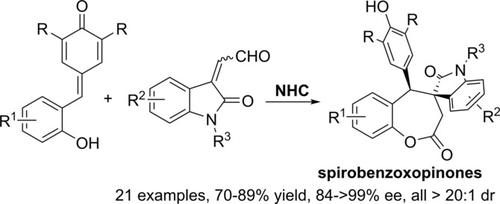
NHC‐Catalyzed Enantioselective [4+3] Cycloaddition of Ortho‐Hydroxyphenyl Substituted Para‐Quinone Methides with Isatin‐Derived Enals
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-05-11 , DOI: 10.1002/adsc.201800337 Wenjun Li 1 , Huijun Yuan 1 , Zhantao Liu 1 , Zhongyin Zhang 1 , Yuyu Cheng 2 , Pengfei Li 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-05-11 , DOI: 10.1002/adsc.201800337 Wenjun Li 1 , Huijun Yuan 1 , Zhantao Liu 1 , Zhongyin Zhang 1 , Yuyu Cheng 2 , Pengfei Li 2
Affiliation
|
The first enantioselective cycloaddition of ortho‐hydroxyphenyl substituted para‐quinone methides has been established by employing isatin‐derived enals as suitable 3C‐synthons under chiral N‐heterocyclic carbene catalysis. By using this strategy, biologically important ϵ‐lactones have been prepared in good yields (up to 89%) and excellent asymmetric inductions (up to >99% ee, >20:1 dr). Notably, this methodology opens a direct [4+3] annulation entry for the asymmetric synthesis of spirobenzoxopinones bearing an oxindole moiety connected through the spirocenter.
中文翻译:

NHC催化的对羟基苯基对映体甲基对苯二酚与对映体衍生的对映体的对映体选择性[4 + 3]环加成反应。
的第一对映选择性环加成邻-羟基苯基取代的对位-quinone甲基化物已经通过使用靛红衍生的enals如在手性合适的3C-合成子建立ñ -杂环卡宾催化。通过使用该策略,已制备出具有生物学重要意义的β-内酯,收率高(高达89%)和出色的不对称诱导(ee高达> 99%,dr> 20:1)。值得注意的是,该方法为不对称合成带有通过螺中心连接的羟吲哚基团的螺苯并氧杂萘酮类化合物的不对称合成开辟了一个直接的[4 + 3]环行入口。
更新日期:2018-05-11
中文翻译:

NHC催化的对羟基苯基对映体甲基对苯二酚与对映体衍生的对映体的对映体选择性[4 + 3]环加成反应。
的第一对映选择性环加成邻-羟基苯基取代的对位-quinone甲基化物已经通过使用靛红衍生的enals如在手性合适的3C-合成子建立ñ -杂环卡宾催化。通过使用该策略,已制备出具有生物学重要意义的β-内酯,收率高(高达89%)和出色的不对称诱导(ee高达> 99%,dr> 20:1)。值得注意的是,该方法为不对称合成带有通过螺中心连接的羟吲哚基团的螺苯并氧杂萘酮类化合物的不对称合成开辟了一个直接的[4 + 3]环行入口。



























 京公网安备 11010802027423号
京公网安备 11010802027423号