当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Planar‐Chiral Rhodium(III) Catalyst with a Sterically Demanding Cyclopentadienyl Ligand and Its Application in the Enantioselective Synthesis of Dihydroisoquinolones
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-17 , DOI: 10.1002/anie.201801703 Evgeniya A. Trifonova 1 , Nikita M. Ankudinov 1 , Andrey A. Mikhaylov 1 , Denis A. Chusov 1 , Yulia V. Nelyubina 1 , Dmitry S. Perekalin 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-17 , DOI: 10.1002/anie.201801703 Evgeniya A. Trifonova 1 , Nikita M. Ankudinov 1 , Andrey A. Mikhaylov 1 , Denis A. Chusov 1 , Yulia V. Nelyubina 1 , Dmitry S. Perekalin 1
Affiliation
|
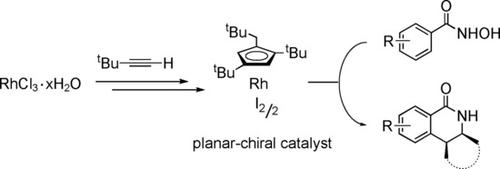
The rapid development of enantioselective C−H activation reactions has created a demand for new types of catalysts. Herein, we report the synthesis of a novel planar‐chiral rhodium catalyst [(C5H2tBu2CH2tBu)RhI2]2 in two steps from commercially available [(cod)RhCl]2 and tert‐butylacetylene. Pure enantiomers of the catalyst were obtained through separation of its diastereomeric adducts with natural (S)‐proline. The catalyst promoted enantioselective reactions of aryl hydroxamic acids with strained alkenes to give dihydroisoquinolones in high yields (up to 97 %) and with good stereoselectivity (up to 95 % ee).
中文翻译:

具有空间需求的环戊二烯基配体的平面手性铑(III)催化剂及其在二氢异喹诺酮对映选择性合成中的应用
对映选择性CH活化反应的迅速发展产生了对新型催化剂的需求。在这里,我们报告了从市售[(cod)RhCl] 2和叔丁基乙炔分两步合成新型平面手性铑催化剂[(C 5 H 2 t Bu 2 CH 2 t Bu)RhI 2 ] 2的合成。催化剂的纯对映体是通过将其非对映体加合物与天然(S脯氨酸 催化剂促进了芳基异羟肟酸与应变烯烃的对映选择性反应,从而以高收率(最高97%)和良好的立体选择性(最高ee 95%)提供了二氢异喹诺酮。
更新日期:2018-04-17
中文翻译:

具有空间需求的环戊二烯基配体的平面手性铑(III)催化剂及其在二氢异喹诺酮对映选择性合成中的应用
对映选择性CH活化反应的迅速发展产生了对新型催化剂的需求。在这里,我们报告了从市售[(cod)RhCl] 2和叔丁基乙炔分两步合成新型平面手性铑催化剂[(C 5 H 2 t Bu 2 CH 2 t Bu)RhI 2 ] 2的合成。催化剂的纯对映体是通过将其非对映体加合物与天然(S脯氨酸 催化剂促进了芳基异羟肟酸与应变烯烃的对映选择性反应,从而以高收率(最高97%)和良好的立体选择性(最高ee 95%)提供了二氢异喹诺酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号