当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accessing Frustrated Lewis Pair Chemistry from a Spectroscopically Stable and Classical Lewis Acid‐Base Adduct
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-17 , DOI: 10.1002/anie.201802385 Timothy C. Johnstone 1 , Gabriel N. J. H. Wee 1 , Douglas W. Stephan 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-17 , DOI: 10.1002/anie.201802385 Timothy C. Johnstone 1 , Gabriel N. J. H. Wee 1 , Douglas W. Stephan 1
Affiliation
|
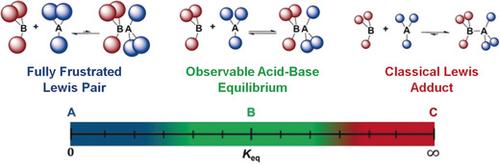
B(C6F5)3 and P(MeNCH2CH2)3N form a classical Lewis adduct, (C6F5)3BP(MeNCH2CH2)3N. Although (C6F5)3BP(MeNCH2CH2)3N does not exhibit spectroscopic evidence of dissociation into its constituent acid and base, products of frustrated Lewis pair (FLP) addition reactions are seen with PhNCO, PhCH2N3, PhNSO, and CO2. Computational studies show that thermal access to the dissociated acid and base permits FLP reactivity to proceed. These results demonstrate that FLP reactivity extends across the entire continuum of equilibria governing Lewis acid‐base adducts.
中文翻译:

从光谱稳定的经典路易斯酸碱加合物中获得沮丧的路易斯对化学
B(C 6 ˚F 5)3和P(MeNCH 2 CH 2)3 Ñ形成经典路易斯加合物,(C 6 ˚F 5)3 BP(MeNCH 2 CH 2)3 N.虽然(C 6 ˚F 5)3 BP (MeNCH 2 CH 2)3 N没有表现出解离成其组成的酸和碱的光谱证据,人们发现与PhNCO,PhCH 2 N 3,PhNSO和CO 2发生受挫的路易斯对(FLP)加成反应的产物。计算研究表明,与解离的酸和碱的热接触使FLP反应性得以进行。这些结果表明,FLP反应性遍及控制路易斯酸碱加合物的整个平衡过程。
更新日期:2018-04-17
中文翻译:

从光谱稳定的经典路易斯酸碱加合物中获得沮丧的路易斯对化学
B(C 6 ˚F 5)3和P(MeNCH 2 CH 2)3 Ñ形成经典路易斯加合物,(C 6 ˚F 5)3 BP(MeNCH 2 CH 2)3 N.虽然(C 6 ˚F 5)3 BP (MeNCH 2 CH 2)3 N没有表现出解离成其组成的酸和碱的光谱证据,人们发现与PhNCO,PhCH 2 N 3,PhNSO和CO 2发生受挫的路易斯对(FLP)加成反应的产物。计算研究表明,与解离的酸和碱的热接触使FLP反应性得以进行。这些结果表明,FLP反应性遍及控制路易斯酸碱加合物的整个平衡过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号