当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile Synthesis of Chiral Cyclic Ureas through Hydrogenation of 2‐Hydroxypyrimidine/Pyrimidin‐2(1H)‐one Tautomers
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-17 , DOI: 10.1002/anie.201801485 Guang-Shou Feng 1, 2 , Mu-Wang Chen 1 , Lei Shi 1 , Yong-Gui Zhou 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-17 , DOI: 10.1002/anie.201801485 Guang-Shou Feng 1, 2 , Mu-Wang Chen 1 , Lei Shi 1 , Yong-Gui Zhou 1
Affiliation
|
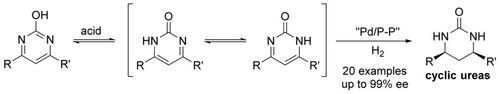
A facile access to optically active cyclic ureas was developed through palladium‐catalyzed asymmetric hydrogenation of pyrimidines containing tautomeric hydroxy group with up to 99 % ee. Mechanistic studies indicated that reaction pathway proceed through hydrogenation of C=N of the oxo tautomer pyrimidin‐2(1H)‐one, acid‐catalyzed isomerization of enamine–imine, and hydrogenation of imine pathway. In addition, the chiral cyclic ureas are readily converted into useful chiral 1,3‐diamine and thiourea derivatives without loss of optical purity.
中文翻译:

通过2-羟基嘧啶/嘧啶-2(1H)-1互变异构体加氢轻松合成手性环状尿素
通过钯催化不对称氢化的嘧啶类化合物,可以容易地获得旋光性环状脲,其中嘧啶类的互变异构羟基含量高达ee的99%。机理研究表明,反应途径是通过羰基互变异构体嘧啶2(1 H)-1的C = N氢化,烯胺-亚胺的酸催化异构化和亚胺途径的氢化而进行的。此外,手性环状脲很容易转化为有用的手性1,3-二胺和硫脲衍生物,而不会降低光学纯度。
更新日期:2018-04-17
中文翻译:

通过2-羟基嘧啶/嘧啶-2(1H)-1互变异构体加氢轻松合成手性环状尿素
通过钯催化不对称氢化的嘧啶类化合物,可以容易地获得旋光性环状脲,其中嘧啶类的互变异构羟基含量高达ee的99%。机理研究表明,反应途径是通过羰基互变异构体嘧啶2(1 H)-1的C = N氢化,烯胺-亚胺的酸催化异构化和亚胺途径的氢化而进行的。此外,手性环状脲很容易转化为有用的手性1,3-二胺和硫脲衍生物,而不会降低光学纯度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号