当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbazole-, Aspidofractinine-, and Aspidocarpamine-Type Alkaloids from Pleiocarpa pycnantha
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00958 Joseph T. Ndongo 1, 2 , Joséphine N. Mbing 3 , Aymeric Monteillier 4 , Michel F. Tala 2 , Michael Rütten 5 , Daniel Mombers 5 , Muriel Cuendet 4 , Dieudonné E. Pegnyemb 3 , Birger Dittrich 5 , Hartmut Laatsch 2
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00958 Joseph T. Ndongo 1, 2 , Joséphine N. Mbing 3 , Aymeric Monteillier 4 , Michel F. Tala 2 , Michael Rütten 5 , Daniel Mombers 5 , Muriel Cuendet 4 , Dieudonné E. Pegnyemb 3 , Birger Dittrich 5 , Hartmut Laatsch 2
Affiliation
|
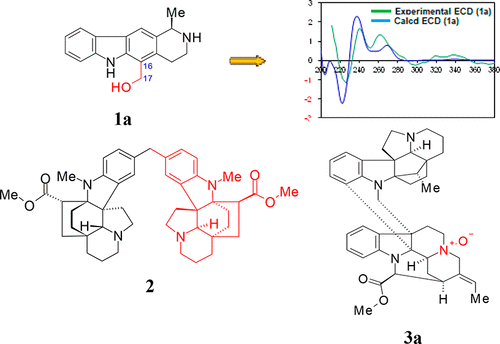
Three new alkaloids, janetinine (1a), pleiokomenine A (2), and huncaniterine B (3a), and 13 known compounds, pleiomutinine (3b), huncaniterine A (3c), 1-carbomethoxy-β-carboline (4), evoxanthine (5), deformyltalbotine acid lactone (6), pleiocarpamine (7), N4-methyl-10-hydroxygeissoschizol (8), spegatrine (9), neosarpagine (10), aspidofractinine (11), N1-methylkopsinin (12), pleiocarpine (13), and N1-methylkopsinin-N4-oxide (14), were isolated from the stem bark of Pleiocarpa pycnantha. Janetinine (1a) is a carbazole alkaloid; in pleiokomenine A (2), two aspidofractinine-type alkaloids are bridged by a methylene unit in an unprecedented way, and huncaniterine B (3a) is a pleiocarpamine–aspidofractinine-type dimer. The structures and relative configurations of these compounds were elucidated on the basis of NMR and MS analyses. Their absolute configurations were defined by means of experimental and calculated ECD data, and additionally, the structures of 5 and 13 were determined by single crystal X-ray diffraction. Compounds 1a, 2, 3b, 4, 6, 9, and 12 displayed cancer chemopreventive properties through either quinone reductase induction (CD = 30.7, 30.2, 29.9, 43.5, and 36.7 μM for 1a, 4, 6, 9, and 12, respectively) and/or NF-κB inhibition with IC50 values of 13.1, 8.4, 9.4, and 8.8 μM for 2, 3b, 6, and 12, respectively.
中文翻译:

苦竹的咔唑类,天冬氨酸吗啡类和天冬酰胺类生物碱
三种新的生物碱,茉莉碱(1a),pleiokomenine A(2)和huncaniterine B(3a),以及13种已知化合物pleiomutinine(3b),huncaniterine A(3c),1-carbomethoxy-β-carboline(4),evoxanthine (5),甲磺基talbotine酸内酯(6),多巴果胺(7),N 4-甲基-10-羟基geissoschizol(8),斜柏碱(9),新帕金森(10),天冬氨酸吗啡碱(11),N 1-甲基甲鱼碱(12) ,多卡洛平(13),和N 1-甲基kopsinin- N 4-氧化物(14),是从苦竹的茎皮中分离出来的。珍妮宁(1a)是咔唑生物碱;在pleiokomenine A(2)中,两个aspidofractinine型生物碱以前所未有的方式被亚甲基单元桥接,而huncaniterine B(3a)是pleiocarpamine-aspidofractinine型二聚体。根据NMR和MS分析,阐明了这些化合物的结构和相对构型。通过实验和计算得出的ECD数据定义了它们的绝对构型,此外,还定义了5和13的结构通过单晶X射线衍射确定。化合物1A,2,图3b,4,6,9,和12通过任一醌还原酶的诱导(显示癌症化学预防性质CD = 30.7,30.2,29.9,43.5,和36.7μM为1A,4,6,9,和12,分别地)和/或NF-κB的抑制带IC 50 13.1,8.4,9.4,和8.8μM的为值2,图3b,6,和12,分别。
更新日期:2018-04-17
中文翻译:

苦竹的咔唑类,天冬氨酸吗啡类和天冬酰胺类生物碱
三种新的生物碱,茉莉碱(1a),pleiokomenine A(2)和huncaniterine B(3a),以及13种已知化合物pleiomutinine(3b),huncaniterine A(3c),1-carbomethoxy-β-carboline(4),evoxanthine (5),甲磺基talbotine酸内酯(6),多巴果胺(7),N 4-甲基-10-羟基geissoschizol(8),斜柏碱(9),新帕金森(10),天冬氨酸吗啡碱(11),N 1-甲基甲鱼碱(12) ,多卡洛平(13),和N 1-甲基kopsinin- N 4-氧化物(14),是从苦竹的茎皮中分离出来的。珍妮宁(1a)是咔唑生物碱;在pleiokomenine A(2)中,两个aspidofractinine型生物碱以前所未有的方式被亚甲基单元桥接,而huncaniterine B(3a)是pleiocarpamine-aspidofractinine型二聚体。根据NMR和MS分析,阐明了这些化合物的结构和相对构型。通过实验和计算得出的ECD数据定义了它们的绝对构型,此外,还定义了5和13的结构通过单晶X射线衍射确定。化合物1A,2,图3b,4,6,9,和12通过任一醌还原酶的诱导(显示癌症化学预防性质CD = 30.7,30.2,29.9,43.5,和36.7μM为1A,4,6,9,和12,分别地)和/或NF-κB的抑制带IC 50 13.1,8.4,9.4,和8.8μM的为值2,图3b,6,和12,分别。











































 京公网安备 11010802027423号
京公网安备 11010802027423号