当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective synthesis of the resveratrol analogue 4,4′-dihydroxy-trans-stilbene and stilbenoids modification by fungal peroxygenases†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1039/c8cy00272j Carmen Aranda 1, 2, 3, 4 , René Ullrich 5, 6, 7, 8 , Jan Kiebist 8, 9, 10 , Katrin Scheibner 8, 9, 10 , José C. del Río 1, 2, 3, 4 , Martin Hofrichter 5, 6, 7, 8 , Angel T. Martínez 2, 4, 11, 12 , Ana Gutiérrez 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1039/c8cy00272j Carmen Aranda 1, 2, 3, 4 , René Ullrich 5, 6, 7, 8 , Jan Kiebist 8, 9, 10 , Katrin Scheibner 8, 9, 10 , José C. del Río 1, 2, 3, 4 , Martin Hofrichter 5, 6, 7, 8 , Angel T. Martínez 2, 4, 11, 12 , Ana Gutiérrez 1, 2, 3, 4
Affiliation
|
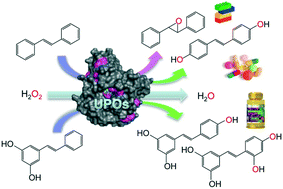
This work gives first evidence that the unspecific peroxygenases (UPOs) from the basidiomycetes Agrocybe aegerita (AaeUPO), Coprinopsis cinerea (rCciUPO) and Marasmius rotula (MroUPO) are able to catalyze the regioselective hydroxylation of trans-stilbene to 4,4′-dihydroxy-trans-stilbene (DHS), a resveratrol (RSV) analogue whose preventive effects on cancer invasion and metastasis have very recently been shown. Nearly complete transformation of substrate (yielding DHS) was achieved with the three enzymes tested, using H2O2 as the only co-substrate, with AaeUPO showing exceptionally higher total turnover number (200 000) than MroUPO (26 000) and rCciUPO (1400). Kinetic studies demonstrated that AaeUPO was the most efficient enzyme catalyzing stilbene dihydroxylation with catalytic efficiencies (kcat/Km) one and two orders of magnitude higher than those of MroUPO and rCciUPO, so that 4-hydroxystilbene appears to be the best UPO substrate reported to date. In contrast, the peroxygenase from the ascomycete Chaetomium globosum (CglUPO) failed to hydroxylate trans-stilbene at the aromatic ring and instead produced the trans-epoxide in the alkenyl moiety. In addition, stilbenoids such as pinosylvin (Pin) and RSV were tested as substrates for the enzymatic synthesis of RSV from Pin and oxyresveratrol (oxyRSV) from both RSV and Pin. Overall, lower conversion rates and regioselectivities compared with trans-stilbene were accomplished by three of the UPOs, and no conversion was observed with CglUPO. The highest amount of RSV (63% of products) and oxyRSV (78%) were again attained with AaeUPO. True peroxygenase activity was demonstrated by incorporation of 18O from H218O2 into the stilbene hydroxylation products. Differences in the number of phenylalanine residues at the heme access channels seems related to differences in aromatic hydroxylation activity, since they would facilitate substrate positioning by aromatic-aromatic interactions. The only ascomycete UPO tested (that of C. globosum) turned out to have the most differing active site (distal side of heme cavity) and reactivity with stilbenes resulting in ethenyl epoxidation instead of aromatic hydroxylation. The above oxyfunctionalizations by fungal UPOs represent a novel and simple alternative to chemical synthesis for the production of DHS, RSV and oxyRSV.
中文翻译:

真菌过氧化酶 对白藜芦醇类似物4,4'-二羟基-反式-二苯乙烯和类苯乙烯类化合物的选择性合成†
这项工作提供了第一个证据来自担子菌的非特异性过氧合酶(不稳定周期轨道)茶树菇(AAE UPO),Coprinopsis孢(R子C ci UPO)和皮rotula(MRO UPO)能的区域选择性催化羟化反式-二苯乙烯至4, 4'-二羟基-反式-二苯乙烯(DHS),一种白藜芦醇(RSV)类似物,最近已显示出其对癌症侵袭和转移的预防作用。使用H 2 O 2作为唯一的底物,与Aae一起测试的三种酶可以实现底物的几乎完全转化(产生DHS)。UPO的总营业额(20万)比Mro UPO(26 000)和r Cci UPO(1400)高得多。动力学研究表明,Aae UPO是最有效的催化二苯乙烯二羟基化的酶,其催化效率(k cat / K m)比Mro UPO和r Cci UPO高一到两个数量级,因此4-羟基二苯乙烯似乎是最有效的酶。迄今为止报告的最佳UPO底物。相比之下,来自子囊壳小球囊壳虫(Cgl UPO)的过氧化酶未能羟化反式-芳环上的-sti烯,而是在烯基部分中产生反式-环氧化物。此外,对类甜菜碱如松果酚(Pinosylvin(Pin)和RSV)作为底物进行酶促合成,包括酶促合成Pin的RSV和氧白藜芦醇(oxyRSV)的RSV和Pin。总体而言,其中三个UPO与反式二苯乙烯相比具有较低的转化率和区域选择性,而Cgl UPO则未观察到转化。RSV(产品63%)和oxyRSV(78%)的最高量再次达到与AAE UPO。通过从H 2 18 O 2中引入18 O证明了真正的过氧化酶活性进入二苯乙烯羟基化产物。血红素进入通道的苯丙氨酸残基数量的差异似乎与芳香族羟基化活性的差异有关,因为它们将通过芳香族-芳香族相互作用促进底物的定位。唯一被测试的子囊菌UPO(球孢梭菌)的活性位点(血红素腔的远端)具有最大的差异,并且与对苯二酚具有反应性,导致乙烯基环氧化而不是芳香族羟基化。真菌UPO的上述氧官能化代表了用于生产DHS,RSV和oxyRSV的化学合成的新颖而简单的替代方法。
更新日期:2018-04-17
中文翻译:

真菌过氧化酶 对白藜芦醇类似物4,4'-二羟基-反式-二苯乙烯和类苯乙烯类化合物的选择性合成†
这项工作提供了第一个证据来自担子菌的非特异性过氧合酶(不稳定周期轨道)茶树菇(AAE UPO),Coprinopsis孢(R子C ci UPO)和皮rotula(MRO UPO)能的区域选择性催化羟化反式-二苯乙烯至4, 4'-二羟基-反式-二苯乙烯(DHS),一种白藜芦醇(RSV)类似物,最近已显示出其对癌症侵袭和转移的预防作用。使用H 2 O 2作为唯一的底物,与Aae一起测试的三种酶可以实现底物的几乎完全转化(产生DHS)。UPO的总营业额(20万)比Mro UPO(26 000)和r Cci UPO(1400)高得多。动力学研究表明,Aae UPO是最有效的催化二苯乙烯二羟基化的酶,其催化效率(k cat / K m)比Mro UPO和r Cci UPO高一到两个数量级,因此4-羟基二苯乙烯似乎是最有效的酶。迄今为止报告的最佳UPO底物。相比之下,来自子囊壳小球囊壳虫(Cgl UPO)的过氧化酶未能羟化反式-芳环上的-sti烯,而是在烯基部分中产生反式-环氧化物。此外,对类甜菜碱如松果酚(Pinosylvin(Pin)和RSV)作为底物进行酶促合成,包括酶促合成Pin的RSV和氧白藜芦醇(oxyRSV)的RSV和Pin。总体而言,其中三个UPO与反式二苯乙烯相比具有较低的转化率和区域选择性,而Cgl UPO则未观察到转化。RSV(产品63%)和oxyRSV(78%)的最高量再次达到与AAE UPO。通过从H 2 18 O 2中引入18 O证明了真正的过氧化酶活性进入二苯乙烯羟基化产物。血红素进入通道的苯丙氨酸残基数量的差异似乎与芳香族羟基化活性的差异有关,因为它们将通过芳香族-芳香族相互作用促进底物的定位。唯一被测试的子囊菌UPO(球孢梭菌)的活性位点(血红素腔的远端)具有最大的差异,并且与对苯二酚具有反应性,导致乙烯基环氧化而不是芳香族羟基化。真菌UPO的上述氧官能化代表了用于生产DHS,RSV和oxyRSV的化学合成的新颖而简单的替代方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号