当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Dihalogenation in Total Synthesis
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1021/acs.accounts.8b00064 Matthew L Landry 1 , Noah Z Burns 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1021/acs.accounts.8b00064 Matthew L Landry 1 , Noah Z Burns 1
Affiliation
|
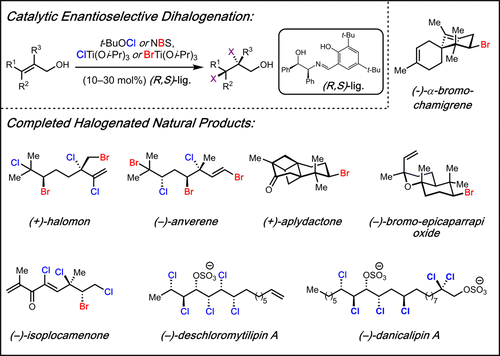
To date, more than 5000 biogenic halogenated molecules have been characterized. This number continues to increase as chemists explore chloride- and bromide-rich marine environments in search of novel bioactive natural products. Naturally occurring organohalogens span nearly all biosynthetic structural classes, exhibit a range of unique biological activities, and have been the subject of numerous investigations. Despite the abundance of and interest in halogenated molecules, enantioselective methods capable of forging carbon–halogen bonds in synthetically relevant contexts remain scarce. Accordingly, syntheses of organohalogens often rely on multistep functional group interconversions to establish carbon–halogen stereocenters. Our group has developed an enantioselective dihalogenation reaction and utilized it in the only reported examples of catalytic enantioselective halogenation in natural product synthesis. In this Account, we describe our laboratory’s development of a method for catalytic, enantioselective dihalogenation and the application of this method to the synthesis of both mono- and polyhalogenated natural products. In the first part, we describe the initial discovery of a TADDOL-mediated dibromination of cinnamyl alcohols. Extension of this reaction to a second-generation system capable of selective bromochlorination, dichlorination, and dibromination is then detailed. This system is capable of controlling the enantioselectivity of dihalide formation, chemoselectivity for polyolefinic substrates, and regioselectivity in the case of bromochlorination. The ability of this method to exert control over regioselectivity of halide delivery permits selective halogenation of electronically nonbiased olefins required for total synthesis.
中文翻译:

全合成中的催化对映选择性二卤化
迄今为止,已经对 5000 多种生物卤化分子进行了表征。随着化学家探索富含氯化物和溴化物的海洋环境以寻找新型生物活性天然产物,这个数字继续增加。天然存在的有机卤素几乎涵盖所有生物合成结构类别,表现出一系列独特的生物活性,并且已成为众多研究的主题。尽管卤化分子丰富且令人感兴趣,但能够在合成相关环境中形成碳-卤素键的对映选择性方法仍然很少。因此,有机卤素的合成通常依赖于多步官能团相互转化来建立碳-卤素立体中心。我们的小组开发了一种对映选择性二卤化反应,并将其用于天然产物合成中催化对映选择性卤化的唯一报道实例中。在本报告中,我们描述了我们实验室开发的一种催化对映选择性二卤化方法,以及该方法在单卤代和多卤代天然产物合成中的应用。在第一部分中,我们描述了 TADDOL 介导的肉桂醇二溴化的初步发现。然后详细介绍了将该反应扩展到能够选择性溴氯化、二氯化和二溴化的第二代系统。该系统能够控制二卤化物形成的对映选择性、聚烯烃底物的化学选择性以及溴氯化情况下的区域选择性。该方法能够控制卤化物输送的区域选择性,从而可以选择性卤化全合成所需的电子无偏烯烃。
更新日期:2018-04-17
中文翻译:

全合成中的催化对映选择性二卤化
迄今为止,已经对 5000 多种生物卤化分子进行了表征。随着化学家探索富含氯化物和溴化物的海洋环境以寻找新型生物活性天然产物,这个数字继续增加。天然存在的有机卤素几乎涵盖所有生物合成结构类别,表现出一系列独特的生物活性,并且已成为众多研究的主题。尽管卤化分子丰富且令人感兴趣,但能够在合成相关环境中形成碳-卤素键的对映选择性方法仍然很少。因此,有机卤素的合成通常依赖于多步官能团相互转化来建立碳-卤素立体中心。我们的小组开发了一种对映选择性二卤化反应,并将其用于天然产物合成中催化对映选择性卤化的唯一报道实例中。在本报告中,我们描述了我们实验室开发的一种催化对映选择性二卤化方法,以及该方法在单卤代和多卤代天然产物合成中的应用。在第一部分中,我们描述了 TADDOL 介导的肉桂醇二溴化的初步发现。然后详细介绍了将该反应扩展到能够选择性溴氯化、二氯化和二溴化的第二代系统。该系统能够控制二卤化物形成的对映选择性、聚烯烃底物的化学选择性以及溴氯化情况下的区域选择性。该方法能够控制卤化物输送的区域选择性,从而可以选择性卤化全合成所需的电子无偏烯烃。











































 京公网安备 11010802027423号
京公网安备 11010802027423号