当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of α‐Synuclein Amyloid Fibril Elongation by Blocking Fibril Ends
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-16 , DOI: 10.1002/anie.201801071 Volodymyr V. Shvadchak 1 , Kseniia Afitska 1 , Dmytro A. Yushchenko 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-16 , DOI: 10.1002/anie.201801071 Volodymyr V. Shvadchak 1 , Kseniia Afitska 1 , Dmytro A. Yushchenko 1, 2
Affiliation
|
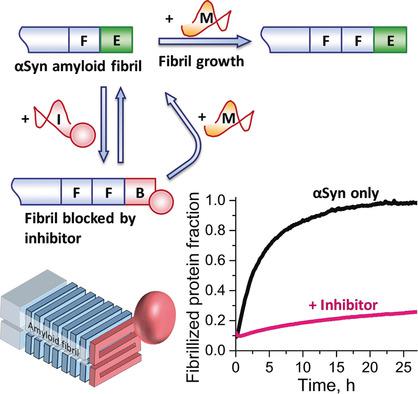
Misfolding of the protein α‐synuclein (αSyn) into amyloid fibrils plays a central role in the development of Parkinson's disease. Most approaches for the inhibition of αSyn fibril formation are based on stabilizing the native monomeric form of the protein or destabilizing the fibrillized misfolded form. They require high concentrations of inhibitor and therefore cannot be easily used for therapies. In this work, we designed an inhibitor (Inh‐β) that selectively binds the growing ends of αSyn fibrils and creates steric hindrance for the binding of monomeric αSyn. This approach permits the inhibition of fibril formation at Inh‐β concentrations (IC50=850 nm) much lower than the concentration of monomeric αSyn. We studied its kinetic mechanism in vitro and identified the reactions that limit inhibition efficiency. It is shown that blocking of αSyn fibril ends is an effective approach to inhibiting fibril growth and provides insights for the development of effective inhibitors of αSyn aggregation.
中文翻译:

阻断原纤维末端抑制α-突触核蛋白淀粉样蛋白原纤维的延伸
蛋白α-突触核蛋白(αSyn)错误折叠成淀粉样原纤维在帕金森氏病的发展中起着重要作用。抑制αSyn原纤维形成的大多数方法是基于稳定蛋白质的天然单体形式或使原纤维化的错误折叠形式不稳定。它们需要高浓度的抑制剂,因此不能轻易用于治疗。在这项工作中,我们设计了一种抑制剂(Inh-β),该抑制剂可选择性地结合αSyn原纤维的生长末端并为单体αSyn的结合产生空间位阻。这种方法可以抑制Inh-β浓度下的原纤维形成(IC 50 = 850 n m)远低于单体αSyn的浓度。我们在体外研究了其动力学机理,并确定了限制抑制效率的反应。结果表明,αSyn原纤维末端的封闭是一种抑制原纤维生长的有效方法,并为开发有效的αSyn聚集抑制剂提供了见识。
更新日期:2018-04-16
中文翻译:

阻断原纤维末端抑制α-突触核蛋白淀粉样蛋白原纤维的延伸
蛋白α-突触核蛋白(αSyn)错误折叠成淀粉样原纤维在帕金森氏病的发展中起着重要作用。抑制αSyn原纤维形成的大多数方法是基于稳定蛋白质的天然单体形式或使原纤维化的错误折叠形式不稳定。它们需要高浓度的抑制剂,因此不能轻易用于治疗。在这项工作中,我们设计了一种抑制剂(Inh-β),该抑制剂可选择性地结合αSyn原纤维的生长末端并为单体αSyn的结合产生空间位阻。这种方法可以抑制Inh-β浓度下的原纤维形成(IC 50 = 850 n m)远低于单体αSyn的浓度。我们在体外研究了其动力学机理,并确定了限制抑制效率的反应。结果表明,αSyn原纤维末端的封闭是一种抑制原纤维生长的有效方法,并为开发有效的αSyn聚集抑制剂提供了见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号