当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iridium‐Catalyzed Sequential Silylation and Borylation of Heteroarenes Based on Regioselective C−H Bond Activation
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-04-16 , DOI: 10.1002/anie.201801229 Masahito Murai 1 , Naoki Nishinaka 1 , Kazuhiko Takai 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-04-16 , DOI: 10.1002/anie.201801229 Masahito Murai 1 , Naoki Nishinaka 1 , Kazuhiko Takai 1
Affiliation
|
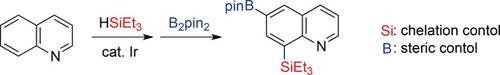
An iridium‐catalyzed regioselective sequential silylation and borylation of heteroarenes was developed, which represents a rare example of unsymmetrical intermolecular C−H bond difunctionalization through the introduction of two different functionalities during a one‐pot transformation. Although the substrate scope for the dehydrogenative silylation of heteroarenes has been limited mainly to electron‐rich five‐membered rings, the current reaction proceeds with both electron‐rich and electron‐deficient heteroarenes with the aid of heteroatom‐directing C−H bond activation. The regioselectivity of the second borylation was controlled by both steric factors and the electronic effect of the silyl group installed in the first step. In combination with the classic cross‐coupling reaction, this method provides rapid access to multisubstituted heteroarenes.
中文翻译:

基于区域选择性CH键活化的铱催化杂芳烃的序列硅烷化和硼化
开发了铱催化的杂芳烃的区域选择性连续甲硅烷基化和硼化,通过一锅转换过程中引入了两个不同的官能团,代表了不对称分子间CH键双官能化的罕见例子。尽管杂芳烃脱氢甲硅烷基化的作用域主要限于富电子五元环,但借助杂原子导向的CH键的活化,当前的反应同时发生于富电子和缺电子的杂芳烃上。第二个硼酸酯化的区域选择性由空间因素和第一步中安装的甲硅烷基的电子效应控制。结合经典的交叉偶联反应,该方法可快速进入多取代的杂芳烃。
更新日期:2018-04-16
中文翻译:

基于区域选择性CH键活化的铱催化杂芳烃的序列硅烷化和硼化
开发了铱催化的杂芳烃的区域选择性连续甲硅烷基化和硼化,通过一锅转换过程中引入了两个不同的官能团,代表了不对称分子间CH键双官能化的罕见例子。尽管杂芳烃脱氢甲硅烷基化的作用域主要限于富电子五元环,但借助杂原子导向的CH键的活化,当前的反应同时发生于富电子和缺电子的杂芳烃上。第二个硼酸酯化的区域选择性由空间因素和第一步中安装的甲硅烷基的电子效应控制。结合经典的交叉偶联反应,该方法可快速进入多取代的杂芳烃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号