Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2018-04-10 , DOI: 10.1016/j.jssc.2018.03.001 P.O. Andreev , L.A. Pimneva
|
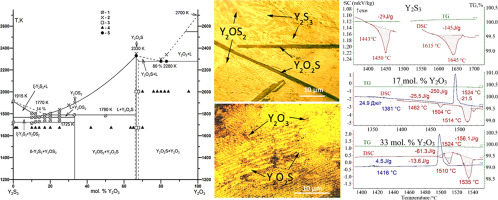
A phase diagram of the Y2S3-Y2O3, system has been defined from 1000 K to melts for the first time; the enthalpies of phase transitions in the systems have been determined. The monoclinic phase δ-Y2S3 (P21/m, a = 1.7523(8) nm, b = 0.4010(9) nm, с = 1.0170(7) nm, β = 98.60(6)°; microhardness H = 411 ± 7 HV) transforms at 1716 ± 7 K to the unquenchable high-temperature phase ξ-Y2S3, ΔН = 29 ± 6 J/g (7.9 KJ/mol) as determined by DSC. The quenching can’t latch the Y2S3-phase. The melting point of Y2S3 is 1888 ± 7 K; ΔН = 150 ± 28 J/g (41.1 KJ/mol). Y2OS2 has a monoclinic structure (P21/c, а = 0.8256(8) nm, b = 0.6879(8) nm, с = 0.6848(8) nm, β = 99.52(6), Н = 491 ± 13 HV) and melts incongruently at 1790 ± 8 K, ΔН = 190 ± 45 J/g (52 KJ/mol) by the scheme Y2OS2 ↔ Y2O2S + L (16 mol% Y2O3). Y2O2S has a hexagonal structure (a = 0.3784(5) nm, c= 0.6584(4) nm, Н = 654 ± 7 HV). Its congruent melting temperature is 2350 ± 40 K as determined by visual polythermal analysis (VPTA). The eutectic formed by Y2S3 and Y2OS2 phases has the composition 14.0 ± 0.5 mol% Y2O3 (0.58Y2S3 + 0.42Y2OS2) and melting temperature 1770 ± 6 K; ΔН = 215 ± 39 J/g. Between Y2O2S and Y2O3 phases, there is a eutectic with the coordinates 80 ± 1 mol% Y2O3 (0.6Y2O2S + 0.4Y2O3) and melting temperature 2150 ± 35 K (VPTA).
中文翻译:

Y 2 S 3 – Y 2 O 3相图和相变焓
Y 2 S 3 -Y 2 O 3系统的相图已从1000 K首次定义为熔融。已经确定了系统中的相变焓。单斜晶相δ-Y 2小号3(P2 1 / M,A = 1.7523(8)纳米,B = 0.4010(9)处,с= 1.0170(7)处,β= 98.60(6)°;显微硬度H =在1716±7 K将难以抑制高温相ξ-Y 411±7 HV)变换2小号3,ΔН= 29±6焦耳/克(7.9 KJ / mol)的通过DSC测定。淬灭不能锁存Y 2 S 3相。Y 2 S 3的熔点是1888±7 K; ΔН= 150±28J / g(41.1KJ / mol)。Y 2 OS 2具有单斜晶结构(P 21 / c,а= 0.8256(8)nm,b = 0.6879(8)nm,с= 0.6848(8)nm,β= 99.52(6),Н= 491±13 HV)和熔体固液异在1790±8 K,ΔН= 190±45焦耳/克(52千焦/摩尔)由方案ÿ 2 OS 2 ↔ý 2 ö 2 S + L(16摩尔%Y 2 ö 3)。Y 2 O 2 S具有六边形结构(a = 0.3784(5)nm,c = 0.6584(4)nm,Н= 654±7 HV)。通过视觉多热分析(VPTA)测定,其全融温度为2350±40K。由Y 2 S形成的共晶3相和Y 2 OS 2相的组成为14.0±0.5mol%的Y 2 O 3(0.58Y 2 S 3 + 0.42Y 2 OS 2),熔融温度为1770± 6K 。ΔН= 215±39 J / g。在Y 2 O 2 S和Y 2 O 3相之间,有一个共晶,坐标为80±1 mol%Y 2 O 3(0.6Y 2 O 2 S + 0.4Y 2 O 3),熔融温度为2150±35 K (VPTA)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号