当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A MIL-101 Composite Doped with Porous Carbon from Tobacco Stem for Enhanced Acetone Uptake at Normal Temperature
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-04-20 , DOI: 10.1021/acs.iecr.8b00393 Denghui Li , Liqing Li , Ruofei Chen , Chunhao Wang , Hailong Li , Haoyang Li
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-04-20 , DOI: 10.1021/acs.iecr.8b00393 Denghui Li , Liqing Li , Ruofei Chen , Chunhao Wang , Hailong Li , Haoyang Li
|
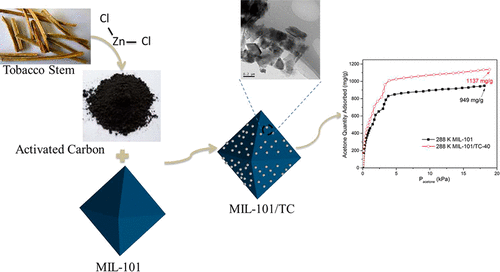
A high-efficiency adsorbent for acetone under normal temperature was prepared with metal–organic framework MIL-101 doped with porous carbon from tobacco stem (MIL-101/TC) by a solvothermal synthesis method. These synthesized composites were characterized and then investigated for acetone adsorption at 288 and 298 K up to 20 kPa. The isosteric heat of adsorption was estimated from adsorption isotherms of acetone vapor. Temperature programmed desorption (TPD) was employed to calculate the activation energies of acetone desorption on MIL-101/TC and MIL-101. Results of characterization showed that MIL-101/TC possessed crystal structure and morphology similar to those of MIL-101. Despite the smaller BET surface area, MIL-101/TC-40 and MIL-101/TC-30 composites had much higher acetone uptakes of 1137 and 1123 mg/g at 18.9 and 18.1 kPa in 288 K, respectively, which increased by 19.8% and 18.3% compared with those of MIL-101 composites. The increase in acetone uptakes was attributed to the associative effect of the enhancement of the surface dispersive forces and the activation of the unsaturated metal sites by TC loading. The isosteric heat of acetone adsorption on MIL-101/TC was much higher than that on MIL-101, and the maximum isosteric adsorption heat on MIL-101/TC-40 was 52 kJ/mol. The activation energy of desorption obtained through TPD ranged from 24.5 to 48.5 kJ/mol. The acetone adsorption isotherms of the composites could be fitted favorably by the L-F and DSLF equations.
中文翻译:

烟草茎中掺有多孔碳的MIL-101复合材料,可提高常温下丙酮的摄取量
采用溶剂热合成法,用烟草茎中的多孔碳(MIL-101 / TC)掺杂的金属有机骨架MIL-101,制备了常温下高效丙酮吸附剂。对这些合成的复合材料进行表征,然后研究其在288 K和298 K下至20 kPa的丙酮吸附率。从丙酮蒸气的吸附等温线估计吸附的等排热。使用程序升温脱附(TPD)来计算MIL-101 / TC和MIL-101上丙酮脱附的活化能。表征结果表明,MIL-101 / TC具有与MIL-101相似的晶体结构和形态。尽管BET表面积较小,但MIL-101 / TC-40和MIL-101 / TC-30复合材料在288 K的18.9 kPa和18.1 kPa处的丙酮吸收量更高,分别为1137和1123 mg / g,与MIL-101复合材料相比分别增长了19.8%和18.3%。丙酮摄入量的增加归因于表面分散力的增强和TC负载对不饱和金属位点的活化的联合作用。MIL-101 / TC上丙酮的等规吸附热远高于MIL-101,而MIL-101 / TC-40上的最大等规吸附热为52 kJ / mol。通过TPD获得的解吸活化能为24.5至48.5 kJ / mol。LF和DSLF方程可以很好地拟合复合材料的丙酮吸附等温线。丙酮摄入量的增加归因于表面分散力的增强和TC负载对不饱和金属位点的活化的联合作用。MIL-101 / TC上丙酮的等规吸附热远高于MIL-101,而MIL-101 / TC-40上的最大等规吸附热为52 kJ / mol。通过TPD获得的解吸活化能为24.5至48.5 kJ / mol。LF和DSLF方程可以很好地拟合复合材料的丙酮吸附等温线。丙酮摄入量的增加归因于表面分散力的增强和TC负载对不饱和金属位点的活化的联合作用。MIL-101 / TC上丙酮的等规吸附热远高于MIL-101,而MIL-101 / TC-40上的最大等规吸附热为52 kJ / mol。通过TPD获得的解吸活化能为24.5至48.5 kJ / mol。LF和DSLF方程可以很好地拟合复合材料的丙酮吸附等温线。5至48.5 kJ / mol。LF和DSLF方程可以很好地拟合复合材料的丙酮吸附等温线。5至48.5 kJ / mol。LF和DSLF方程可以很好地拟合复合材料的丙酮吸附等温线。
更新日期:2018-04-23
中文翻译:

烟草茎中掺有多孔碳的MIL-101复合材料,可提高常温下丙酮的摄取量
采用溶剂热合成法,用烟草茎中的多孔碳(MIL-101 / TC)掺杂的金属有机骨架MIL-101,制备了常温下高效丙酮吸附剂。对这些合成的复合材料进行表征,然后研究其在288 K和298 K下至20 kPa的丙酮吸附率。从丙酮蒸气的吸附等温线估计吸附的等排热。使用程序升温脱附(TPD)来计算MIL-101 / TC和MIL-101上丙酮脱附的活化能。表征结果表明,MIL-101 / TC具有与MIL-101相似的晶体结构和形态。尽管BET表面积较小,但MIL-101 / TC-40和MIL-101 / TC-30复合材料在288 K的18.9 kPa和18.1 kPa处的丙酮吸收量更高,分别为1137和1123 mg / g,与MIL-101复合材料相比分别增长了19.8%和18.3%。丙酮摄入量的增加归因于表面分散力的增强和TC负载对不饱和金属位点的活化的联合作用。MIL-101 / TC上丙酮的等规吸附热远高于MIL-101,而MIL-101 / TC-40上的最大等规吸附热为52 kJ / mol。通过TPD获得的解吸活化能为24.5至48.5 kJ / mol。LF和DSLF方程可以很好地拟合复合材料的丙酮吸附等温线。丙酮摄入量的增加归因于表面分散力的增强和TC负载对不饱和金属位点的活化的联合作用。MIL-101 / TC上丙酮的等规吸附热远高于MIL-101,而MIL-101 / TC-40上的最大等规吸附热为52 kJ / mol。通过TPD获得的解吸活化能为24.5至48.5 kJ / mol。LF和DSLF方程可以很好地拟合复合材料的丙酮吸附等温线。丙酮摄入量的增加归因于表面分散力的增强和TC负载对不饱和金属位点的活化的联合作用。MIL-101 / TC上丙酮的等规吸附热远高于MIL-101,而MIL-101 / TC-40上的最大等规吸附热为52 kJ / mol。通过TPD获得的解吸活化能为24.5至48.5 kJ / mol。LF和DSLF方程可以很好地拟合复合材料的丙酮吸附等温线。5至48.5 kJ / mol。LF和DSLF方程可以很好地拟合复合材料的丙酮吸附等温线。5至48.5 kJ / mol。LF和DSLF方程可以很好地拟合复合材料的丙酮吸附等温线。










































 京公网安备 11010802027423号
京公网安备 11010802027423号