当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Promiscuity of Phenol Hydroxylase from Pseudomonas stutzeri OX1 for the Biosynthesis of Phenolic Compounds
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-04-16 00:00:00 , DOI: 10.1021/acssynbio.8b00067 Jia Wang 1, 2 , Xiaolin Shen 1, 2 , Jian Wang 3 , Yaping Yang 3 , Qipeng Yuan 1, 2 , Yajun Yan 3
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-04-16 00:00:00 , DOI: 10.1021/acssynbio.8b00067 Jia Wang 1, 2 , Xiaolin Shen 1, 2 , Jian Wang 3 , Yaping Yang 3 , Qipeng Yuan 1, 2 , Yajun Yan 3
Affiliation
|
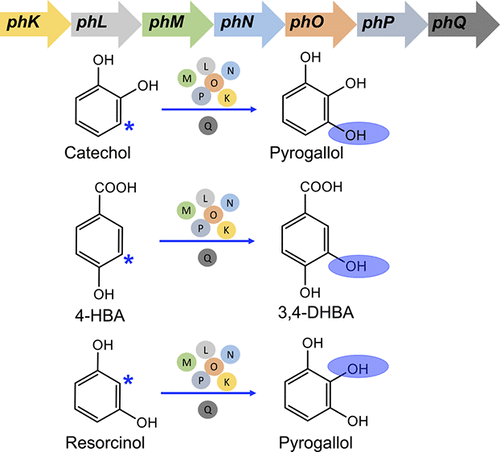
Enzyme promiscuity plays an important role in developing biosynthetic pathways for novel target products. Phenol hydroxylase (PH) from Pseudomonas stutzeri OX1 is capable of ortho-hydroxylation of phenol and cresol isomers into counterpart catechols. A small ferredoxin-like protein PHQ was clustered together with the ph gene cluster in the genome of P. stutzeri OX1, and its function was not known. In this study, we found that the existence of PHQ has a promotion effect on the catalytic efficiency of PH. Then, we tested the substrate range of PH using nine different non-natural substrates. We found that PH was a promiscuous hydroxylase that could catalyze ortho-hydroxylation of several non-natural substrates, including catechol, 4-hydroxybenzoic acid and resorcinol. On this basis, linking the catechol biosynthetic pathway with the hydroxylation reaction catalyzed by PH enabled construction of a novel biosynthetic pathway for the synthesis of pyrogallol. This work not only characterized a well-performed PH, but also provided a promising hydroxylation platform for the production of high-value phenolic compounds.
中文翻译:

探索斯氏假单胞菌OX1中苯酚羟化酶用于酚类化合物生物合成的滥交性
酶混杂在开发新型靶标产品的生物合成途径中起着重要作用。斯图氏假单胞菌OX1中的苯酚羟化酶(PH)能够将苯酚和甲酚异构体进行邻羟基化成为相应的邻苯二酚。一个小的铁氧还蛋白样蛋白PHQ和ph基因簇一起聚在斯图氏假单胞菌的基因组中OX1,其功能尚不清楚。在这项研究中,我们发现PHQ的存在对PH的催化效率具有促进作用。然后,我们使用9种不同的非天然底物测试了PH的底物范围。我们发现PH是一种混杂的羟化酶,可以催化几种非天然底物的邻羟基化,包括邻苯二酚,4-羟基苯甲酸和间苯二酚。在此基础上,将邻苯二酚的生物合成途径与PH催化的羟基化反应联系起来,便可以构建一种新型的合成邻苯三酚的生物合成途径。这项工作不仅表征了良好的PH,而且还为生产高价值酚类化合物提供了有希望的羟基化平台。
更新日期:2018-04-16
中文翻译:

探索斯氏假单胞菌OX1中苯酚羟化酶用于酚类化合物生物合成的滥交性
酶混杂在开发新型靶标产品的生物合成途径中起着重要作用。斯图氏假单胞菌OX1中的苯酚羟化酶(PH)能够将苯酚和甲酚异构体进行邻羟基化成为相应的邻苯二酚。一个小的铁氧还蛋白样蛋白PHQ和ph基因簇一起聚在斯图氏假单胞菌的基因组中OX1,其功能尚不清楚。在这项研究中,我们发现PHQ的存在对PH的催化效率具有促进作用。然后,我们使用9种不同的非天然底物测试了PH的底物范围。我们发现PH是一种混杂的羟化酶,可以催化几种非天然底物的邻羟基化,包括邻苯二酚,4-羟基苯甲酸和间苯二酚。在此基础上,将邻苯二酚的生物合成途径与PH催化的羟基化反应联系起来,便可以构建一种新型的合成邻苯三酚的生物合成途径。这项工作不仅表征了良好的PH,而且还为生产高价值酚类化合物提供了有希望的羟基化平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号