Journal of the American Society for Mass Spectrometry ( IF 3.1 ) Pub Date : 2018-04-16 , DOI: 10.1007/s13361-018-1904-3 Kelly L. Wormwood 1 , Armand Gatien Ngounou Wetie 1 , Marcus Vinicius Gomez 2 , Yue Ju 3 , Paul Kowalski 3 , Marius Mihasan 1, 4 , Costel C. Darie 1
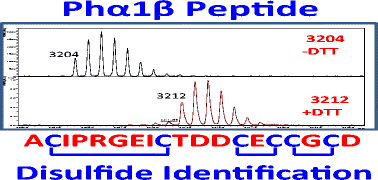
Native Phα1β is a peptide purified from the venom of the armed spider Phoneutria nigriventer that has been shown to have an extensive analgesic effect with fewer side effects than ω-conotoxin MVIIA. Recombinant Phα1β mimics the effects of the native Phα1β. Because of this, it has been suggested that Phα1β may have potential to be used as a therapeutic for controlling persistent pathological pain. The amino acid sequence of Phα1β is known; however, the exact structure and disulfide arrangement has yet to be determined. Determination of the disulfide linkages and exact structure could greatly assist in pharmacological analysis and determination of why this peptide is such an effective analgesic. Here, we used biochemical and mass spectrometry approaches to determine the disulfide linkages present in the recombinant Phα1β peptide. Using a combination of MALDI-MS, direct infusion ESI-MS, and nanoLC-MS/MS analysis of the undigested recombinant Phα1β peptide and digested with AspN, trypsin, or AspN/trypsin, we were able to identify and confirm all six disulfide linkages present in the peptide as Cys1-2, Cys3-4, Cys5-6, Cys7-8, Cys9-10, and Cys11-12. These results were also partially confirmed in the native Phα1β peptide. These experiments provide essential structural information about Phα1β and may assist in providing insight into the peptide’s analgesic effect with very low side effects.
ᅟ
中文翻译:

质谱分析蜘蛛肽Phα1β的结构表征和二硫键赋值
天然Phα1β是从武装蜘蛛Phoneutria nigriventer的毒液中纯化的肽与ω-芋螺毒素MVIIA相比,它具有广泛的镇痛作用,且副作用较少。重组Phα1β模仿天然Phα1β的作用。因此,已经暗示Phα1β可能具有用作控制持续性病理性疼痛的治疗剂的潜力。Phα1β的氨基酸序列是已知的。但是,确切的结构和二硫键的排列尚未确定。确定二硫键和确切的结构可以极大地帮助进行药理学分析和确定这种肽为何如此有效的镇痛剂。在这里,我们使用生化和质谱方法来确定重组Phα1β肽中存在的二硫键。结合使用MALDI-MS,直接输注ESI-MS,和未消化的重组Phα1β肽的nanoLC-MS / MS分析,并用AspN,胰蛋白酶或AspN /胰蛋白酶消化,我们能够鉴定并确认该肽中存在的所有六个二硫键为Cys1-2,Cys3-4,Cys5 -6,Cys7-8,Cys9-10和Cys11-12。这些结果在天然Phα1β肽中也得到了部分证实。这些实验提供了有关Phα1β的基本结构信息,并可能有助于以非常低的副作用深入了解该肽的镇痛作用。

ᅟ











































 京公网安备 11010802027423号
京公网安备 11010802027423号