当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Charge-transfer complexes based on C2v-symmetric benzo[ghi]perylene: comparison of their dynamic and electronic properties with those of D6h-symmetric coronene
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2018-04-16 , DOI: 10.1039/c8qm00112j Yukihiro Yoshida 1, 2, 3, 4, 5 , Shunsuke Tango 5, 6, 7, 8 , Kazuhide Isomura 5, 6, 7, 8 , Yuto Nakamura 5, 6, 7, 8 , Hideo Kishida 5, 6, 7, 8 , Takashi Koretsune 5, 9, 10, 11, 12 , Masafumi Sakata 13, 14, 15, 16, 17 , Yoshiaki Nakano 1, 2, 3, 4, 5 , Hideki Yamochi 1, 2, 3, 4, 5 , Gunzi Saito 5, 18, 19, 20, 21
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2018-04-16 , DOI: 10.1039/c8qm00112j Yukihiro Yoshida 1, 2, 3, 4, 5 , Shunsuke Tango 5, 6, 7, 8 , Kazuhide Isomura 5, 6, 7, 8 , Yuto Nakamura 5, 6, 7, 8 , Hideo Kishida 5, 6, 7, 8 , Takashi Koretsune 5, 9, 10, 11, 12 , Masafumi Sakata 13, 14, 15, 16, 17 , Yoshiaki Nakano 1, 2, 3, 4, 5 , Hideki Yamochi 1, 2, 3, 4, 5 , Gunzi Saito 5, 18, 19, 20, 21
Affiliation
|
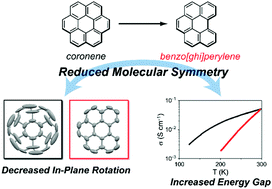
Single crystals of three neutral charge-transfer complexes and a cation radical salt based on a C2v-symmetric polycyclic aromatic hydrocarbon, benzo[ghi]perylene (bper), were obtained. The 1 : 1 complex with 7,7,8,8-tetracyanoquinodimethane (TCNQ) involves DA-type alternating π-columns, whereas the alternating π-columns in the 2 : 1 TCNQ complex are flanked by another bper molecule. The in-plane rotation of bper in the 1 : 1 complex was significantly suppressed compared with that of coronene in (coronene) (TCNQ), which is associated with the lower molecular symmetry of bper. Whereas the 3 : 1 TCNQ complex involves DDA-type alternating π-columns flanked by another bper molecule, bper molecules in the 3 : 1 cation radical salt with Mo6O192− have a columnar structure with a [101]-like charge-ordered pattern associated with intermolecular interactions in the bay region of bper. The dimerisation of charge-rich bper molecules results in an increased energy gap at the Fermi level, and consequently, semiconducting behaviour of the salt has a larger activation energy than that of the isostructural coronene salt in the partially charged state. The lower molecular symmetry of bper also affects the degeneracy of the frontier-orbitals; the energy difference between the HOMO and the HOMO−1 of bper is significantly larger than that of coronene, which is comparable to the intermolecular transfer integrals.
中文翻译:

基于C 2v对称苯并[ ghi ] per的电荷转移配合物:与D 6h对称并戊烯的动态和电子性质比较
获得了三种中性电荷转移络合物和基于C 2v对称多环芳烃苯并[ g ] per(bper)的阳离子自由基盐的单晶。具有7,7,8,8-四氰基喹二甲烷(TCNQ)的1:1络合物涉及DA型交替的π列,而2:1的TCNQ络合物中的交替的π列旁接另一个bper分子。与(coronene)(TCNQ)中的异戊二烯相比,bper在1:1络合物中的面内旋转被显着抑制,这与bper的较低分子对称性有关。3:1 TCNQ络合物涉及DDA型交替的π列,其侧翼是另一个bper分子,bper分子与Mo 6 O在3:1阳离子自由基盐中19 2-具有柱状结构,其具有与bper的海湾区域中的分子间相互作用相关的[101]样电荷排序的模式。富含电荷的bper分子的二聚化导致费米能级上的能隙增加,因此,该盐的半导体行为具有比部分带电状态的同结构异戊二烯盐更大的活化能。bper的较低分子对称性也影响边界轨道的简并性。bper的HOMO和HOMO-1之间的能量差显着大于苯并戊烯,这与分子间转移积分相当。
更新日期:2018-05-31
中文翻译:

基于C 2v对称苯并[ ghi ] per的电荷转移配合物:与D 6h对称并戊烯的动态和电子性质比较
获得了三种中性电荷转移络合物和基于C 2v对称多环芳烃苯并[ g ] per(bper)的阳离子自由基盐的单晶。具有7,7,8,8-四氰基喹二甲烷(TCNQ)的1:1络合物涉及DA型交替的π列,而2:1的TCNQ络合物中的交替的π列旁接另一个bper分子。与(coronene)(TCNQ)中的异戊二烯相比,bper在1:1络合物中的面内旋转被显着抑制,这与bper的较低分子对称性有关。3:1 TCNQ络合物涉及DDA型交替的π列,其侧翼是另一个bper分子,bper分子与Mo 6 O在3:1阳离子自由基盐中19 2-具有柱状结构,其具有与bper的海湾区域中的分子间相互作用相关的[101]样电荷排序的模式。富含电荷的bper分子的二聚化导致费米能级上的能隙增加,因此,该盐的半导体行为具有比部分带电状态的同结构异戊二烯盐更大的活化能。bper的较低分子对称性也影响边界轨道的简并性。bper的HOMO和HOMO-1之间的能量差显着大于苯并戊烯,这与分子间转移积分相当。











































 京公网安备 11010802027423号
京公网安备 11010802027423号