当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sustainable Disposal of Cr(VI): Adsorption–Reduction Strategy for Treating Textile Wastewaters with Amino-Functionalized Boehmite Hazardous Solid Wastes
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-04-15 00:00:00 , DOI: 10.1021/acssuschemeng.8b00640 Hailin Zhang 1, 2 , Ping Li 1 , Zheming Wang 3 , Wen Wen Cui 1, 2 , Yang Zhang 1 , Ying Zhang 1 , Shili Zheng 1 , Yi Zhang 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-04-15 00:00:00 , DOI: 10.1021/acssuschemeng.8b00640 Hailin Zhang 1, 2 , Ping Li 1 , Zheming Wang 3 , Wen Wen Cui 1, 2 , Yang Zhang 1 , Ying Zhang 1 , Shili Zheng 1 , Yi Zhang 1
Affiliation
|
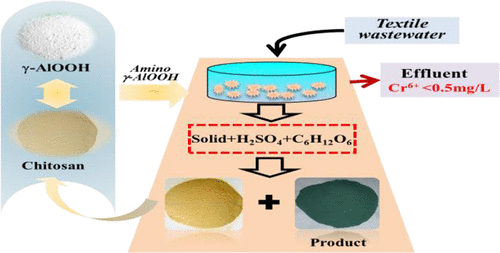
The present work reports a sustainable adsorption–reduction strategy for disposal of Cr(VI)-bearing wastes. By cross-linking between boehmite and chitosan to prepare amino-functionalized boehmite adsorbents, Cr(VI) could be adsorbed from the Cr(VI)-containing solutions by the adsorbents with the maximum adsorption capacity of 120.2 mg/g, which was improved about 3 times compared to that of boehmite. Adsorption mechanism showed that the Cr(VI) was adsorbed by complexing with −NH2 bonds and exchanging with −OH groups of adsorbents. About 31% Cr(VI) was simultaneously reduced to Cr(III) by the reactive −OH and −NH2 electron acceptor of adsorbents. After adsorption, the Cr(VI/III) and boehmite of adsorbents were dissolved in 25% H2SO4 solution, the remaining Cr(VI) was reduced to Cr(III) with 100% reduction yield, and the CrxAl2–x(OH)2y(SO4)8–y·nH2O was prepared as product, while the insoluble chitosan was recycled in a closed loop. In application, column experiments results showed that the γ-AlOOH hazardous wastes could effectively dispose the Cr(VI)-containing textile wastewaters, and aluminum and chromium in the wastes could be comprehensively used to prepare chromium–aluminum tanning agent. The developed “waste-treating-waste” method leads to minimum pollutant emission and avoids the complex post-treatment problems.
中文翻译:

Cr(VI)的可持续处置:吸附-还原策略,用于氨基官能化勃姆石危险固体废物处理纺织品废水
本工作报告了一种可持续的吸附减少战略,用于处理含Cr(VI)的废物。通过勃姆石和壳聚糖之间的交联制备氨基官能化勃姆石吸附剂,Cr(VI)可以从含Cr(VI)的溶液中被吸附剂吸附,最大吸附容量为120.2 mg / g。是勃姆石的3倍。吸附机理表明Cr(VI)通过与-NH 2键络合并与-OH基团交换而被吸附。通过吸附剂的反应性-OH和-NH 2电子受体,同时将约31%的Cr(VI)还原为Cr(III)。吸附后,将吸附剂的Cr(VI / III)和勃姆石溶解在25%H 2 SO 4中溶液中,剩余的Cr(VI)以100%的还原率还原为Cr(III),并制备了Cr x Al 2– x(OH)2 y(SO 4)8– y · n H 2 O ,而不溶性壳聚糖则在闭环中循环使用。在应用中,柱实验结果表明,γ-AlOOH危险废物可以有效地处理含Cr(VI)的纺织废水,并且废物中的铝和铬可以综合用于制备铬铝制革剂。先进的“废物处理-废物”方法可将污染物排放降至最低,并避免了复杂的后处理问题。
更新日期:2018-04-15
中文翻译:

Cr(VI)的可持续处置:吸附-还原策略,用于氨基官能化勃姆石危险固体废物处理纺织品废水
本工作报告了一种可持续的吸附减少战略,用于处理含Cr(VI)的废物。通过勃姆石和壳聚糖之间的交联制备氨基官能化勃姆石吸附剂,Cr(VI)可以从含Cr(VI)的溶液中被吸附剂吸附,最大吸附容量为120.2 mg / g。是勃姆石的3倍。吸附机理表明Cr(VI)通过与-NH 2键络合并与-OH基团交换而被吸附。通过吸附剂的反应性-OH和-NH 2电子受体,同时将约31%的Cr(VI)还原为Cr(III)。吸附后,将吸附剂的Cr(VI / III)和勃姆石溶解在25%H 2 SO 4中溶液中,剩余的Cr(VI)以100%的还原率还原为Cr(III),并制备了Cr x Al 2– x(OH)2 y(SO 4)8– y · n H 2 O ,而不溶性壳聚糖则在闭环中循环使用。在应用中,柱实验结果表明,γ-AlOOH危险废物可以有效地处理含Cr(VI)的纺织废水,并且废物中的铝和铬可以综合用于制备铬铝制革剂。先进的“废物处理-废物”方法可将污染物排放降至最低,并避免了复杂的后处理问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号