Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic insights into the inhibition and size effects of graphene oxide nanosheets on the aggregation of an amyloid-β peptide fragment†
Nanoscale ( IF 5.8 ) Pub Date : 2018-04-16 00:00:00 , DOI: 10.1039/c8nr01041b Yujie Chen 1, 2, 3, 4, 5 , Zihan Chen 1, 2, 3, 4, 5 , Yunxiang Sun 1, 2, 3, 4, 5 , Jiangtao Lei 1, 2, 3, 4, 5 , Guanghong Wei 1, 2, 3, 4, 5
Nanoscale ( IF 5.8 ) Pub Date : 2018-04-16 00:00:00 , DOI: 10.1039/c8nr01041b Yujie Chen 1, 2, 3, 4, 5 , Zihan Chen 1, 2, 3, 4, 5 , Yunxiang Sun 1, 2, 3, 4, 5 , Jiangtao Lei 1, 2, 3, 4, 5 , Guanghong Wei 1, 2, 3, 4, 5
Affiliation
|
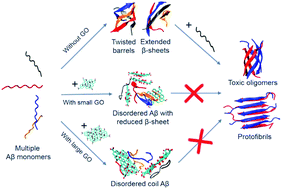
The aggregation of amyloid-β (Aβ), which involves the formation of small oligomers and mature fibrils, has received considerable attention in the past few decades due to its close link with Alzheimer's disease (AD). The inhibition of β-sheet formation has been considered as the primary therapeutic strategy for AD. In this respect, graphene oxide (GO) has gained significant attention because of its high solubility, good biocompatibility and inhibitory effect on the aggregation of Aβ and the 33–42 fragment (Aβ33–42). However, the inhibitory mechanism at the atomic level remains elusive. Herein, we investigated the oligomerization of Aβ33–42 by performing replica exchange molecular dynamics simulations on four Aβ33–42 peptide chains in the absence and presence of two different sizes of GO. Our simulations show that isolated Aβ33–42 can form fibril-prone extended β-sheets and barrel-like structures, whereas they are suppressed in the presence of GO nanosheets. Our data reveal that GO inhibits Aβ33–42 oligomerization by making Aβ33–42 peptides separate from each other through strong interactions with M35. With the same total number of atoms, GO120 displays better inhibitory effect than GO60 by providing a larger effective contact surface area. This study provides the molecular mechanism of GO in inhibiting the aggregation of Aβ33–42, which might offer a theoretical insight into the design of drugs against AD at the atomic level.
中文翻译:

关于氧化石墨烯纳米片对淀粉样β肽片段的聚集的抑制作用和尺寸影响的机理见解†
淀粉样β(Aβ)的聚集涉及小寡聚物和成熟原纤维的形成,由于其与阿尔茨海默氏病(AD)的紧密联系,在过去的几十年中受到了相当大的关注。抑制β-折叠的形成已被认为是AD的主要治疗策略。在这方面,氧化石墨烯(GO)因其高溶解度,良好的生物相容性以及对Aβ和33-42片段(Aβ33-42)聚集的抑制作用而备受关注。但是,在原子水平上的抑制机制仍然难以捉摸。在这里,我们通过对四个Aβ33-42进行复制交换分子动力学模拟来研究Aβ33-42的低聚在缺少和存在两种不同大小的GO的情况下形成肽链。我们的模拟表明,分离的Aβ33-42可以形成原纤维倾向于延伸的β-折叠和桶状结构,而在GO纳米片的存在下它们会被抑制。我们的数据显示,GO通过使Aβ33-42肽通过与M35的强相互作用而彼此分离,从而抑制Aβ33-42的寡聚。在相同的原子总数下,GO 120通过提供更大的有效接触表面积而显示出比GO 60更好的抑制效果。这项研究提供了GO抑制Aβ33-42聚集的分子机制。,这可能会为在原子水平上抗AD药物的设计提供理论上的见识。
更新日期:2018-04-16
中文翻译:

关于氧化石墨烯纳米片对淀粉样β肽片段的聚集的抑制作用和尺寸影响的机理见解†
淀粉样β(Aβ)的聚集涉及小寡聚物和成熟原纤维的形成,由于其与阿尔茨海默氏病(AD)的紧密联系,在过去的几十年中受到了相当大的关注。抑制β-折叠的形成已被认为是AD的主要治疗策略。在这方面,氧化石墨烯(GO)因其高溶解度,良好的生物相容性以及对Aβ和33-42片段(Aβ33-42)聚集的抑制作用而备受关注。但是,在原子水平上的抑制机制仍然难以捉摸。在这里,我们通过对四个Aβ33-42进行复制交换分子动力学模拟来研究Aβ33-42的低聚在缺少和存在两种不同大小的GO的情况下形成肽链。我们的模拟表明,分离的Aβ33-42可以形成原纤维倾向于延伸的β-折叠和桶状结构,而在GO纳米片的存在下它们会被抑制。我们的数据显示,GO通过使Aβ33-42肽通过与M35的强相互作用而彼此分离,从而抑制Aβ33-42的寡聚。在相同的原子总数下,GO 120通过提供更大的有效接触表面积而显示出比GO 60更好的抑制效果。这项研究提供了GO抑制Aβ33-42聚集的分子机制。,这可能会为在原子水平上抗AD药物的设计提供理论上的见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号