当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aggregated Aβ1-42 Is Selectively Toxic for Neurons, Whereas Glial Cells Produce Mature Fibrils with Low Toxicity in Drosophila
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-04-12 , DOI: 10.1016/j.chembiol.2018.03.006 Maria Jonson , Sofie Nyström , Alexander Sandberg , Marcus Carlback , Wojciech Michno , Jörg Hanrieder , Annika Starkenberg , K. Peter R. Nilsson , Stefan Thor , Per Hammarström
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-04-12 , DOI: 10.1016/j.chembiol.2018.03.006 Maria Jonson , Sofie Nyström , Alexander Sandberg , Marcus Carlback , Wojciech Michno , Jörg Hanrieder , Annika Starkenberg , K. Peter R. Nilsson , Stefan Thor , Per Hammarström
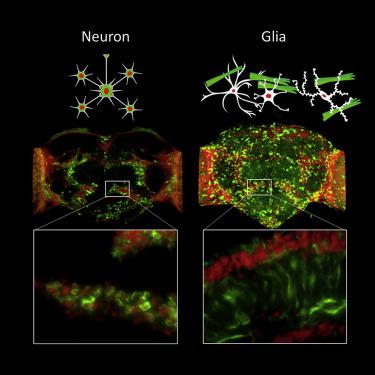
|
The basis for selective vulnerability of certain cell types for misfolded proteins (MPs) in neurodegenerative diseases is largely unknown. This knowledge is crucial for understanding disease progression in relation to MPs spreading in the CNS. We assessed this issue inDrosophilaby cell-specific expression of human Aβ1-42 associated with Alzheimer's disease. Expression of Aβ1-42 in various neurons resulted in concentration-dependent severe neurodegenerative phenotypes, and intraneuronal ring-tangle-like aggregates with immature fibril properties when analyzed by aggregate-specific ligands. Unexpectedly, expression of Aβ1-42 from a pan-glial driver produced a mild phenotype despite massive brain load of Aβ1-42 aggregates, even higher than in the strongest neuronal driver. Glial cells formed more mature fibrous aggregates, morphologically distinct from aggregates found in neurons, and was mainly extracellular. Our findings implicate that Aβ1-42 cytotoxicity is both cell and aggregate morphotype dependent.
中文翻译:

聚集的Aβ1-42对神经元有选择性毒性,而胶质细胞在果蝇中产生低毒性的成熟原纤维。
某些细胞类型对神经退行性疾病中错误折叠的蛋白质(MPs)的选择性脆弱性的基础在很大程度上是未知的。该知识对于了解与MP在CNS中传播有关的疾病进展至关重要。我们在果蝇中与阿尔茨海默氏病相关的人Aβ1-42的细胞特异性表达中评估了这个问题。Aβ1-42在各种神经元中的表达导致浓度依赖性严重的神经变性表型,以及通过聚集体特异性配体分析时具有未成熟原纤维特性的神经内环缠结样聚集体。出乎意料的是,尽管大脑中大量的Aβ1-42聚集体,但泛神经胶质细胞驱动器中Aβ1-42的表达仍产生了轻度的表型,甚至比最强的神经元驱动器还要高。胶质细胞形成更成熟的纤维状聚集体,形态上与神经元中发现的聚集体不同,并且主要在细胞外。我们的发现暗示Aβ1-42细胞毒性既是细胞形态的,也是聚集体形态型的。
更新日期:2018-05-17
中文翻译:

聚集的Aβ1-42对神经元有选择性毒性,而胶质细胞在果蝇中产生低毒性的成熟原纤维。
某些细胞类型对神经退行性疾病中错误折叠的蛋白质(MPs)的选择性脆弱性的基础在很大程度上是未知的。该知识对于了解与MP在CNS中传播有关的疾病进展至关重要。我们在果蝇中与阿尔茨海默氏病相关的人Aβ1-42的细胞特异性表达中评估了这个问题。Aβ1-42在各种神经元中的表达导致浓度依赖性严重的神经变性表型,以及通过聚集体特异性配体分析时具有未成熟原纤维特性的神经内环缠结样聚集体。出乎意料的是,尽管大脑中大量的Aβ1-42聚集体,但泛神经胶质细胞驱动器中Aβ1-42的表达仍产生了轻度的表型,甚至比最强的神经元驱动器还要高。胶质细胞形成更成熟的纤维状聚集体,形态上与神经元中发现的聚集体不同,并且主要在细胞外。我们的发现暗示Aβ1-42细胞毒性既是细胞形态的,也是聚集体形态型的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号