Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Diacetoxylation of Disubstituted 1,2,3-Triazoles through Palladium-Catalyzed C–H Activation
Synlett ( IF 1.7 ) Pub Date : 2018-04-12 , DOI: 10.1055/s-0036-1591564 Huiling Cheng 1 , Yubo Jiang 1 , Jianhua Yang 1 , Fen Zhao 1 , Yaowen Liu 1 , Fang Luo 1
Synlett ( IF 1.7 ) Pub Date : 2018-04-12 , DOI: 10.1055/s-0036-1591564 Huiling Cheng 1 , Yubo Jiang 1 , Jianhua Yang 1 , Fen Zhao 1 , Yaowen Liu 1 , Fang Luo 1
Affiliation
|
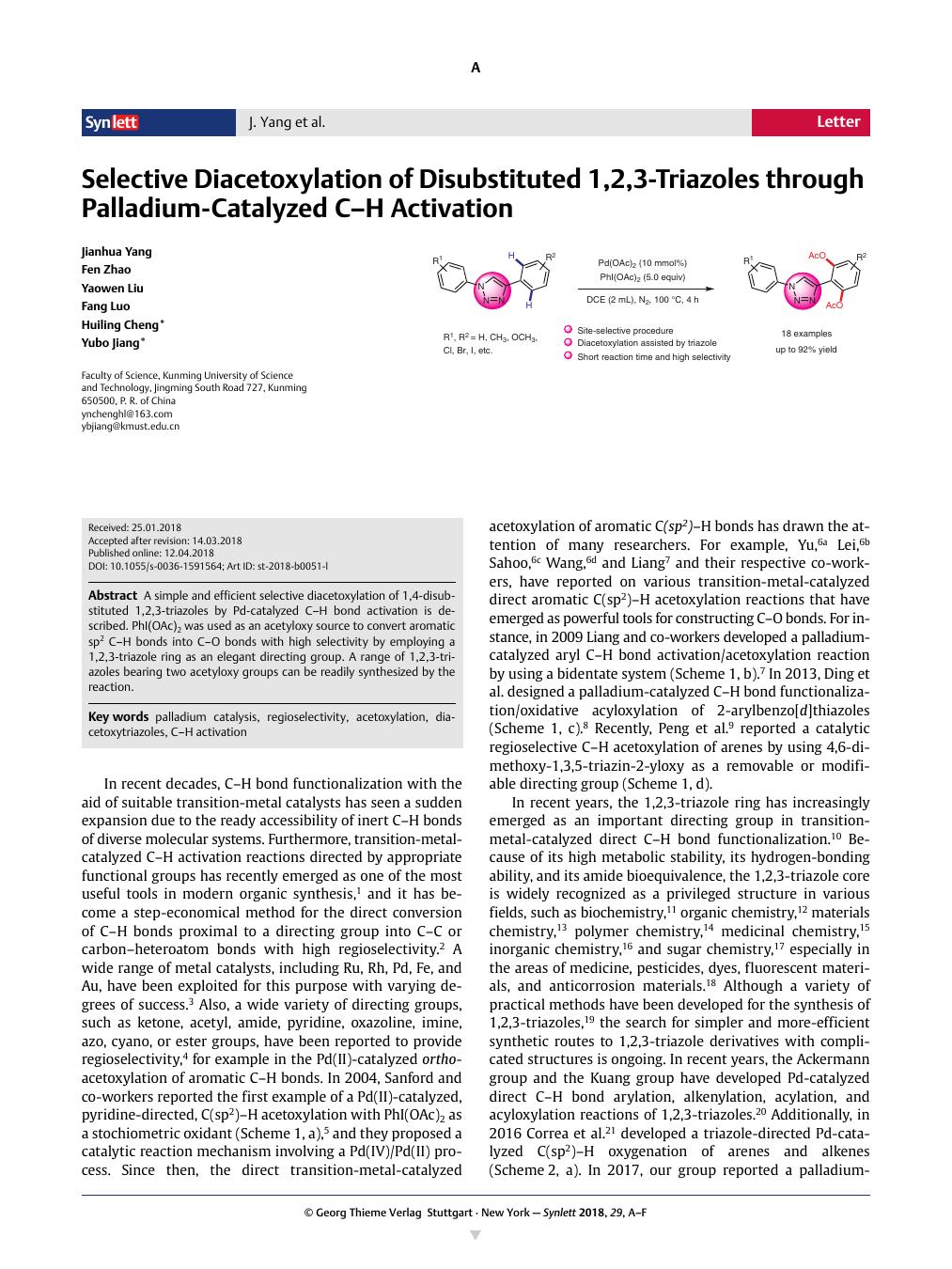
A simple and efficient selective diacetoxylation of 1,4-disubstituted 1,2,3-triazoles by Pd-catalyzed C–H bond activation is described. PhI(OAc) 2 was used as an acetyloxy source to convert aromatic sp 2 C–H bonds into C–O bonds with high selectivity by employing a 1,2,3-triazole ring as an elegant directing group. A range of 1,2,3-triazoles bearing two acetyloxy groups can be readily synthesized by the reaction.
中文翻译:

通过钯催化的 C-H 活化选择性双乙酰氧基化双取代 1,2,3-三唑
描述了通过 Pd 催化的 C-H 键活化对 1,4-二取代的 1,2,3-三唑进行简单有效的选择性二乙酰氧基化。PhI(OAc) 2 被用作乙酰氧基源,通过采用 1,2,3-三唑环作为优雅的导向基团,将芳香 sp 2 C-H 键以高选择性转化为 C-O 键。通过该反应可以很容易地合成一系列带有两个乙酰氧基的 1,2,3-三唑。
更新日期:2018-04-12
中文翻译:

通过钯催化的 C-H 活化选择性双乙酰氧基化双取代 1,2,3-三唑
描述了通过 Pd 催化的 C-H 键活化对 1,4-二取代的 1,2,3-三唑进行简单有效的选择性二乙酰氧基化。PhI(OAc) 2 被用作乙酰氧基源,通过采用 1,2,3-三唑环作为优雅的导向基团,将芳香 sp 2 C-H 键以高选择性转化为 C-O 键。通过该反应可以很容易地合成一系列带有两个乙酰氧基的 1,2,3-三唑。









































 京公网安备 11010802027423号
京公网安备 11010802027423号