当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Holistic Approach to Partial Covalent Interactions in Protein Structure Prediction and Design with Rosetta.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-04-19 , DOI: 10.1021/acs.jcim.7b00398 Steven A Combs 1 , Benjamin K Mueller 1 , Jens Meiler 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-04-19 , DOI: 10.1021/acs.jcim.7b00398 Steven A Combs 1 , Benjamin K Mueller 1 , Jens Meiler 1
Affiliation
|
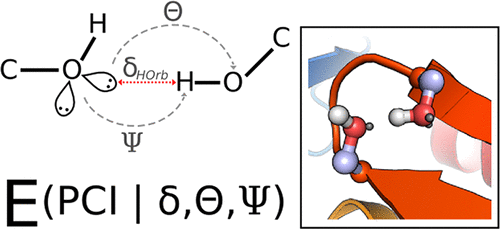
Partial covalent interactions (PCIs) in proteins, which include hydrogen bonds, salt bridges, cation-π, and π-π interactions, contribute to thermodynamic stability and facilitate interactions with other biomolecules. Several score functions have been developed within the Rosetta protein modeling framework that identify and evaluate these PCIs through analyzing the geometry between participating atoms. However, we hypothesize that PCIs can be unified through a simplified electron orbital representation. To test this hypothesis, we have introduced orbital based chemical descriptors for PCIs into Rosetta, called the PCI score function. Optimal geometries for the PCIs are derived from a statistical analysis of high-quality protein structures obtained from the Protein Data Bank (PDB), and the relative orientation of electron deficient hydrogen atoms and electron-rich lone pair or π orbitals are evaluated. We demonstrate that nativelike geometries of hydrogen bonds, salt bridges, cation-π, and π-π interactions are recapitulated during minimization of protein conformation. The packing density of tested protein structures increased from the standard score function from 0.62 to 0.64, closer to the native value of 0.70. Overall, rotamer recovery improved when using the PCI score function (75%) as compared to the standard Rosetta score function (74%). The PCI score function represents an improvement over the standard Rosetta score function for protein model scoring; in addition, it provides a platform for future directions in the analysis of small molecule to protein interactions, which depend on partial covalent interactions.
中文翻译:

利用Rosetta对蛋白质结构预测和设计中部分共价相互作用的整体方法。
蛋白质中的部分共价相互作用(PCI),包括氢键,盐桥,阳离子-π和π-π相互作用,有助于热力学稳定性并促进与其他生物分子的相互作用。已在Rosetta蛋白建模框架内开发了几种评分功能,这些功能通过分析参与原子之间的几何结构来识别和评估这些PCI。但是,我们假设可以通过简化的电子轨道表示来统一PCI。为了检验该假设,我们将基于轨道的PCI的化学描述符引入Rosetta,称为PCI评分函数。PCI的最佳几何结构来自对从蛋白质数据库(PDB)获得的高质量蛋白质结构的统计分析,并评估了缺电子氢原子与富电子孤对或π轨道的相对取向。我们证明了氢键,盐桥,阳离子-π和π-π相互作用的自然几何形状在最小化蛋白质构象的过程中得到了概括。测试的蛋白质结构的堆积密度从标准评分函数从0.62增加到0.64,更接近自然值0.70。总体而言,与标准的Rosetta评分功能(74%)相比,使用PCI评分功能(75%)可提高旋转异构体的回收率。PCI评分功能代表了针对蛋白质模型评分的标准Rosetta评分功能的改进;此外,它为分析小分子与蛋白质之间的相互作用提供了一个平台,该分析依赖于部分共价相互作用。
更新日期:2018-04-11
中文翻译:

利用Rosetta对蛋白质结构预测和设计中部分共价相互作用的整体方法。
蛋白质中的部分共价相互作用(PCI),包括氢键,盐桥,阳离子-π和π-π相互作用,有助于热力学稳定性并促进与其他生物分子的相互作用。已在Rosetta蛋白建模框架内开发了几种评分功能,这些功能通过分析参与原子之间的几何结构来识别和评估这些PCI。但是,我们假设可以通过简化的电子轨道表示来统一PCI。为了检验该假设,我们将基于轨道的PCI的化学描述符引入Rosetta,称为PCI评分函数。PCI的最佳几何结构来自对从蛋白质数据库(PDB)获得的高质量蛋白质结构的统计分析,并评估了缺电子氢原子与富电子孤对或π轨道的相对取向。我们证明了氢键,盐桥,阳离子-π和π-π相互作用的自然几何形状在最小化蛋白质构象的过程中得到了概括。测试的蛋白质结构的堆积密度从标准评分函数从0.62增加到0.64,更接近自然值0.70。总体而言,与标准的Rosetta评分功能(74%)相比,使用PCI评分功能(75%)可提高旋转异构体的回收率。PCI评分功能代表了针对蛋白质模型评分的标准Rosetta评分功能的改进;此外,它为分析小分子与蛋白质之间的相互作用提供了一个平台,该分析依赖于部分共价相互作用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号