Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0035-0 Matthew B Johnson 1, 2 , Xingshen Sun 3, 4, 5 , Andrew Kodani 1, 2 , Rebeca Borges-Monroy 1, 2 , Kelly M Girskis 1, 2 , Steven C Ryu 1, 2 , Peter P Wang 1, 2 , Komal Patel 6 , Dilenny M Gonzalez 1, 2 , Yu Mi Woo 7 , Ziying Yan 3, 4, 5 , Bo Liang 3, 4, 5 , Richard S Smith 1, 2 , Manavi Chatterjee 6 , Daniel Coman 8, 9, 10 , Xenophon Papademetris 9, 10, 11 , Lawrence H Staib 10, 11, 12 , Fahmeed Hyder 8, 9, 10, 11 , Joseph B Mandeville 13 , P Ellen Grant 14 , Kiho Im 14 , Hojoong Kwak 7 , John F Engelhardt 3, 4, 5 , Christopher A Walsh 1, 2 , Byoung-Il Bae 1, 2, 6
Nature ( IF 50.5 ) Pub Date : 2018-04-01 , DOI: 10.1038/s41586-018-0035-0 Matthew B Johnson 1, 2 , Xingshen Sun 3, 4, 5 , Andrew Kodani 1, 2 , Rebeca Borges-Monroy 1, 2 , Kelly M Girskis 1, 2 , Steven C Ryu 1, 2 , Peter P Wang 1, 2 , Komal Patel 6 , Dilenny M Gonzalez 1, 2 , Yu Mi Woo 7 , Ziying Yan 3, 4, 5 , Bo Liang 3, 4, 5 , Richard S Smith 1, 2 , Manavi Chatterjee 6 , Daniel Coman 8, 9, 10 , Xenophon Papademetris 9, 10, 11 , Lawrence H Staib 10, 11, 12 , Fahmeed Hyder 8, 9, 10, 11 , Joseph B Mandeville 13 , P Ellen Grant 14 , Kiho Im 14 , Hojoong Kwak 7 , John F Engelhardt 3, 4, 5 , Christopher A Walsh 1, 2 , Byoung-Il Bae 1, 2, 6
Affiliation
|
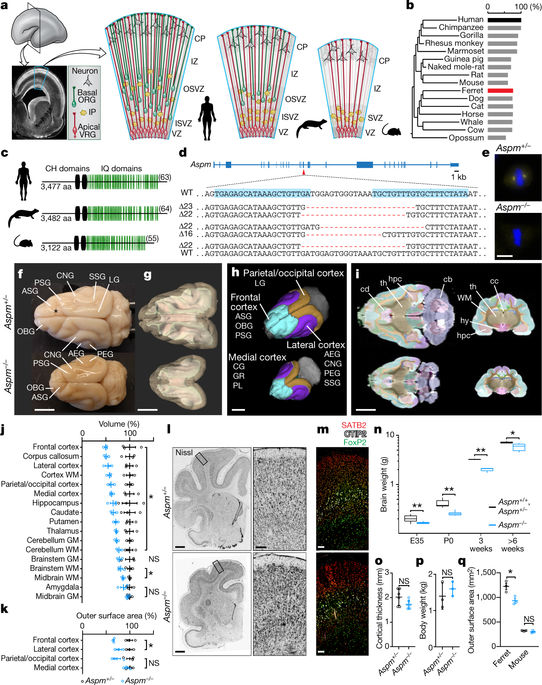
The human cerebral cortex is distinguished by its large size and abundant gyrification, or folding. However, the evolutionary mechanisms that drive cortical size and structure are unknown. Although genes that are essential for cortical developmental expansion have been identified from the genetics of human primary microcephaly (a disorder associated with reduced brain size and intellectual disability)1, studies of these genes in mice, which have a smooth cortex that is one thousand times smaller than the cortex of humans, have provided limited insight. Mutations in abnormal spindle-like microcephaly-associated (ASPM), the most common recessive microcephaly gene, reduce cortical volume by at least 50% in humans2–4, but have little effect on the brains of mice5–9; this probably reflects evolutionarily divergent functions of ASPM10,11. Here we used genome editing to create a germline knockout of Aspm in the ferret (Mustela putorius furo), a species with a larger, gyrified cortex and greater neural progenitor cell diversity12–14 than mice, and closer protein sequence homology to the human ASPM protein. Aspm knockout ferrets exhibit severe microcephaly (25–40% decreases in brain weight), reflecting reduced cortical surface area without significant change in cortical thickness, as has been found in human patients3,4, suggesting that loss of ‘cortical units’ has occurred. The cortex of fetal Aspm knockout ferrets displays a very large premature displacement of ventricular radial glial cells to the outer subventricular zone, where many resemble outer radial glia, a subtype of neural progenitor cells that are essentially absent in mice and have been implicated in cerebral cortical expansion in primates12–16. These data suggest an evolutionary mechanism by which ASPM regulates cortical expansion by controlling the affinity of ventricular radial glial cells for the ventricular surface, thus modulating the ratio of ventricular radial glial cells, the most undifferentiated cell type, to outer radial glia, a more differentiated progenitor.In a ferret model, the microcephaly-associated gene Aspm regulates cortical expansion by controlling the transition of ventricular radial glial cells to more differentiated cell types.
中文翻译:

Aspm 敲除雪貂揭示了控制大脑皮层大小的进化机制
人类大脑皮层以其大尺寸和丰富的回旋或折叠而著称。然而,驱动皮质大小和结构的进化机制尚不清楚。尽管已经从人类原发性小头畸形(一种与大脑体积缩小和智力残疾相关的疾病)的遗传学中发现了对皮质发育扩张至关重要的基因1,但在小鼠中对这些基因进行了研究,这些基因的平滑皮质是一千倍比人类的皮层还小,提供了有限的洞察力。异常纺锤样小头畸形相关 (ASPM) 是最常见的隐性小头畸形基因,其突变可使人类的皮层体积减少至少 50%2-4,但对小鼠的大脑几乎没有影响5-9;这可能反映了 ASPM10,11 在进化上的不同功能。在这里,我们使用基因组编辑在雪貂 (Mustela putorius furo) 中创建了 Aspm 的种系敲除,该物种比小鼠具有更大的旋转皮质和更大的神经祖细胞多样性 12-14,并且蛋白质序列同源性更接近人类 ASPM 蛋白. Aspm 敲除雪貂表现出严重的小头畸形(脑重量减少 25-40%),这反映了皮质表面积减少而皮质厚度没有显着变化,正如在人类患者中发现的那样 3,4,这表明已经发生了“皮质单位”的丧失。胎儿 Aspm 敲除雪貂的皮层显示出非常大的心室放射状胶质细胞过早移位到外脑室下区,其中许多类似于外放射状胶质细胞,一种神经祖细胞亚型,在小鼠中基本上不存在,并且与灵长类动物的大脑皮层扩张有关12-16。这些数据表明了一种进化机制,ASPM 通过控制心室放射状胶质细胞对心室表面的亲和力来调节皮质扩张,从而调节心室放射状胶质细胞(最未分化的细胞类型)与外放射状胶质细胞(分化程度更高)的比例。祖细胞。在雪貂模型中,小头畸形相关基因 Aspm 通过控制心室放射状胶质细胞向分化程度更高的细胞类型的转变来调节皮质扩张。
更新日期:2018-04-01
中文翻译:

Aspm 敲除雪貂揭示了控制大脑皮层大小的进化机制
人类大脑皮层以其大尺寸和丰富的回旋或折叠而著称。然而,驱动皮质大小和结构的进化机制尚不清楚。尽管已经从人类原发性小头畸形(一种与大脑体积缩小和智力残疾相关的疾病)的遗传学中发现了对皮质发育扩张至关重要的基因1,但在小鼠中对这些基因进行了研究,这些基因的平滑皮质是一千倍比人类的皮层还小,提供了有限的洞察力。异常纺锤样小头畸形相关 (ASPM) 是最常见的隐性小头畸形基因,其突变可使人类的皮层体积减少至少 50%2-4,但对小鼠的大脑几乎没有影响5-9;这可能反映了 ASPM10,11 在进化上的不同功能。在这里,我们使用基因组编辑在雪貂 (Mustela putorius furo) 中创建了 Aspm 的种系敲除,该物种比小鼠具有更大的旋转皮质和更大的神经祖细胞多样性 12-14,并且蛋白质序列同源性更接近人类 ASPM 蛋白. Aspm 敲除雪貂表现出严重的小头畸形(脑重量减少 25-40%),这反映了皮质表面积减少而皮质厚度没有显着变化,正如在人类患者中发现的那样 3,4,这表明已经发生了“皮质单位”的丧失。胎儿 Aspm 敲除雪貂的皮层显示出非常大的心室放射状胶质细胞过早移位到外脑室下区,其中许多类似于外放射状胶质细胞,一种神经祖细胞亚型,在小鼠中基本上不存在,并且与灵长类动物的大脑皮层扩张有关12-16。这些数据表明了一种进化机制,ASPM 通过控制心室放射状胶质细胞对心室表面的亲和力来调节皮质扩张,从而调节心室放射状胶质细胞(最未分化的细胞类型)与外放射状胶质细胞(分化程度更高)的比例。祖细胞。在雪貂模型中,小头畸形相关基因 Aspm 通过控制心室放射状胶质细胞向分化程度更高的细胞类型的转变来调节皮质扩张。











































 京公网安备 11010802027423号
京公网安备 11010802027423号