当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical reduction of carbon dioxide with a molecular polypyridyl nickel complex†
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2018-04-11 00:00:00 , DOI: 10.1039/c8se00027a Lauren E. Lieske 1, 2, 3, 4 , Arnold L. Rheingold 4, 5, 6, 7 , Charles W. Machan 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2018-04-11 00:00:00 , DOI: 10.1039/c8se00027a Lauren E. Lieske 1, 2, 3, 4 , Arnold L. Rheingold 4, 5, 6, 7 , Charles W. Machan 1, 2, 3, 4
Affiliation
|
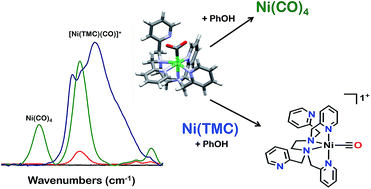
The synthesis and reactivity of a molecular nickel(II) complex 1 with the polypyridyl ligand framework N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) under electrochemically reducing conditions in the presence of CO2 is reported. Cyclic voltammetry (CV), infrared spectroelectrochemistry (IR-SEC) and electrolysis experiments suggest this Ni complex is competent at mediating the two-electron reduction of CO2 to CO and H2O with phenol as an added proton donor, but is subsequently prone to rapid degradation as Ni(CO)4. This deleterious pathway was shown to be mitigated by the inclusion of the CO scavenger [Ni(TMC)]2+, although this requires stoichiometric inclusion for each catalyst turnover and does not significantly improve the observed catalytic current densities.
中文翻译:

用分子聚吡啶镍配合物电化学还原二氧化碳†
据报道,在电化学还原条件下,在CO 2存在下,分子镍(II)配合物1与聚吡啶基配体骨架N,N,N ',N'-四(2-吡啶基甲基)乙二胺(TPEN)的合成和反应性。循环伏安法(CV),红外光谱电化学(IR-SEC)和电解实验表明,该Ni配合物能以酚作为添加的质子供体,介导将CO 2还原为CO 2和H 2 O的两电子还原,但随后容易发生迅速降解为Ni(CO)4。该有害途径显示出通过包含CO清除剂[Ni(TMC)] 2+得以缓解,尽管这对于每种催化剂周转量都需要化学计量的加入,并且并不能显着提高所观察到的催化电流密度。
更新日期:2018-04-11
中文翻译:

用分子聚吡啶镍配合物电化学还原二氧化碳†
据报道,在电化学还原条件下,在CO 2存在下,分子镍(II)配合物1与聚吡啶基配体骨架N,N,N ',N'-四(2-吡啶基甲基)乙二胺(TPEN)的合成和反应性。循环伏安法(CV),红外光谱电化学(IR-SEC)和电解实验表明,该Ni配合物能以酚作为添加的质子供体,介导将CO 2还原为CO 2和H 2 O的两电子还原,但随后容易发生迅速降解为Ni(CO)4。该有害途径显示出通过包含CO清除剂[Ni(TMC)] 2+得以缓解,尽管这对于每种催化剂周转量都需要化学计量的加入,并且并不能显着提高所观察到的催化电流密度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号