当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stability and anti-inflammatory activity of the reduction-resistant curcumin analog, 2,6-dimethyl-curcumin.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-05-02 , DOI: 10.1039/c8ob00639c Akil I Joseph 1 , Rebecca L Edwards , Paula B Luis , Sai Han Presley , Ned A Porter , Claus Schneider
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-05-02 , DOI: 10.1039/c8ob00639c Akil I Joseph 1 , Rebecca L Edwards , Paula B Luis , Sai Han Presley , Ned A Porter , Claus Schneider
Affiliation
|
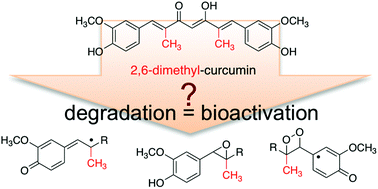
The efficacy of the curry spice compound curcumin as a natural anti-inflammatory agent is limited by its rapid reductive metabolism in vivo. A recent report described a novel synthetic derivative, 2,6-dimethyl-curcumin, with increased stability against reduction in vitro and in vivo. It is also known that curcumin is unstable at physiological pH in vitro and undergoes rapid autoxidative transformation. Since the oxidation products may contribute to the biological effects of curcumin, we tested oxidative stability of 2,6-dimethyl-curcumin in buffer (pH 7.5). The rate of degradation was similar to curcumin. The degradation products were identified as a one-carbon chain-shortened alcohol, vanillin, and two isomeric epoxides that underwent cleavage to vanillin and a corresponding hydroxylated cleavage product. 2,6-Dimethyl-curcumin was more potent than curcumin in inhibiting NF-κB activity but less potent in inhibiting expression of cyclooxygenase-2 in LPS-activated RAW264.7 cells. 2,6-Dimethyl-curcumin and some of its degradation products covalently bound to a peptide that contains the redox-sensitive cysteine of IKKβ kinase, the activating kinase upstream of NF-κB, providing a mechanism for the anti-inflammatory activity. In RAW264.7 cells vanillin, the chain-shortened alcohol, and reduced 2,6-dimethyl-curcumin were detected as major metabolites. These studies provide new insight into the oxidative transformation mechanism of curcumin and related compounds. The products resulting from oxidative transformation contribute to the anti-inflammatory activity of 2,6-dimethyl-curcumin in addition to its enhanced resistance against enzymatic reduction.
中文翻译:

抗还原姜黄素类似物 2,6-二甲基姜黄素的稳定性和抗炎活性。
咖喱香料复合姜黄素作为天然抗炎剂的功效因其在体内的快速还原代谢而受到限制。最近的一份报告描述了一种新型合成衍生物 2,6-二甲基姜黄素,其在体外和体内的还原稳定性均有所提高。还已知姜黄素在体外生理pH下不稳定并经历快速的自氧化转化。由于氧化产物可能有助于姜黄素的生物效应,因此我们测试了 2,6-二甲基姜黄素在缓冲液(pH 7.5)中的氧化稳定性。降解速度与姜黄素相似。降解产物被鉴定为一碳链缩短的醇、香草醛和两种异构环氧化物,它们裂解为香草醛和相应的羟基化裂解产物。在 LPS 激活的 RAW264.7 细胞中,2,6-Dimethyl-curcumin 在抑制 NF-κB 活性方面比姜黄素更有效,但在抑制 cyclooxygenase-2 表达方面较姜黄素更有效。 2,6-二甲基姜黄素及其一些降解产物与含有 IKKβ 激酶(NF-κB 上游激活激酶)氧化还原敏感半胱氨酸的肽共价结合,提供了抗炎活性机制。在 RAW264.7 细胞中,香草醛、链缩短的酒精和还原的 2,6-二甲基姜黄素被检测为主要代谢物。这些研究为姜黄素和相关化合物的氧化转化机制提供了新的见解。氧化转化产生的产物除了增强对酶还原的抵抗力之外,还有助于 2,6-二甲基姜黄素的抗炎活性。
更新日期:2018-04-11
中文翻译:

抗还原姜黄素类似物 2,6-二甲基姜黄素的稳定性和抗炎活性。
咖喱香料复合姜黄素作为天然抗炎剂的功效因其在体内的快速还原代谢而受到限制。最近的一份报告描述了一种新型合成衍生物 2,6-二甲基姜黄素,其在体外和体内的还原稳定性均有所提高。还已知姜黄素在体外生理pH下不稳定并经历快速的自氧化转化。由于氧化产物可能有助于姜黄素的生物效应,因此我们测试了 2,6-二甲基姜黄素在缓冲液(pH 7.5)中的氧化稳定性。降解速度与姜黄素相似。降解产物被鉴定为一碳链缩短的醇、香草醛和两种异构环氧化物,它们裂解为香草醛和相应的羟基化裂解产物。在 LPS 激活的 RAW264.7 细胞中,2,6-Dimethyl-curcumin 在抑制 NF-κB 活性方面比姜黄素更有效,但在抑制 cyclooxygenase-2 表达方面较姜黄素更有效。 2,6-二甲基姜黄素及其一些降解产物与含有 IKKβ 激酶(NF-κB 上游激活激酶)氧化还原敏感半胱氨酸的肽共价结合,提供了抗炎活性机制。在 RAW264.7 细胞中,香草醛、链缩短的酒精和还原的 2,6-二甲基姜黄素被检测为主要代谢物。这些研究为姜黄素和相关化合物的氧化转化机制提供了新的见解。氧化转化产生的产物除了增强对酶还原的抵抗力之外,还有助于 2,6-二甲基姜黄素的抗炎活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号