当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereodivergent synthesis of 5-aminopipecolic acids and application in the preparation of a cyclic RGD peptidomimetic as a nanomolar αVβ3 integrin ligand†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-11 00:00:00 , DOI: 10.1039/c8ob00534f Lorenzo Sernissi 1, 2, 3, 4 , Luciano Ricci 1, 2, 3, 4 , Dina Scarpi 1, 2, 3, 4 , Francesca Bianchini 4, 5, 6, 7, 8 , Daniela Arosio 4, 9, 10, 11 , Alessandro Contini 4, 12, 13, 14 , Ernesto G. Occhiato 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-11 00:00:00 , DOI: 10.1039/c8ob00534f Lorenzo Sernissi 1, 2, 3, 4 , Luciano Ricci 1, 2, 3, 4 , Dina Scarpi 1, 2, 3, 4 , Francesca Bianchini 4, 5, 6, 7, 8 , Daniela Arosio 4, 9, 10, 11 , Alessandro Contini 4, 12, 13, 14 , Ernesto G. Occhiato 1, 2, 3, 4
Affiliation
|
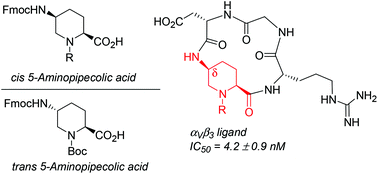
A stereodivergent strategy was devised to obtain enantiopure cis and trans 5-aminopipecolic acids (5-APAs) in suitably protected forms to be employed in peptide synthesis as conformationally constrained α- and δ-amino acids. The cis isomer was used as a δ-amino acid to construct a cyclic RGD-containing peptidomimetic, the ability of which to compete with biotinylated vitronectin for binding with the isolated αVβ3 integrin was measured (IC50 = 4.2 ± 0.9 nM). A complete 1H NMR and computational conformational analysis was performed to elucidate the reasons for the high affinity of this cyclic peptidomimetic in comparison with cilengitide.
中文翻译:

5- aminopipecolic酸Stereodivergent合成和制备的环状RGD肽模拟物作为纳摩尔α应用V β 3整联蛋白配†
设计了立体发散策略,以得到适当保护形式的对映纯顺式和反式5-氨基哌酸(5-APA),以构象约束的α-和δ-氨基酸形式用于肽合成。的顺式异构体被用作δ氨基酸,构建环状含RGD的肽模拟物,其中,以与生物素化的玻连蛋白竞争与所述分离α结合的能力V β 3(IC整合测定50 = 4.2±0.9 nm)的。进行了完整的1 H NMR和计算构象分析,以阐明与cilengitide相比,该环状拟肽具有高亲和力的原因。
更新日期:2018-04-11
中文翻译:

5- aminopipecolic酸Stereodivergent合成和制备的环状RGD肽模拟物作为纳摩尔α应用V β 3整联蛋白配†
设计了立体发散策略,以得到适当保护形式的对映纯顺式和反式5-氨基哌酸(5-APA),以构象约束的α-和δ-氨基酸形式用于肽合成。的顺式异构体被用作δ氨基酸,构建环状含RGD的肽模拟物,其中,以与生物素化的玻连蛋白竞争与所述分离α结合的能力V β 3(IC整合测定50 = 4.2±0.9 nm)的。进行了完整的1 H NMR和计算构象分析,以阐明与cilengitide相比,该环状拟肽具有高亲和力的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号