当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NFSI-participated intermolecular aminoazidation of alkene through iron catalysis†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-11 00:00:00 , DOI: 10.1039/c8ob00699g Bowen Lei 1, 2, 3, 4, 5 , Xiaojiao Wang 1, 2, 3, 4, 5 , Lifang Ma 1, 2, 3, 4, 5 , Yan Li 1, 2, 3, 4, 5 , Ziyuan Li 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-11 00:00:00 , DOI: 10.1039/c8ob00699g Bowen Lei 1, 2, 3, 4, 5 , Xiaojiao Wang 1, 2, 3, 4, 5 , Lifang Ma 1, 2, 3, 4, 5 , Yan Li 1, 2, 3, 4, 5 , Ziyuan Li 1, 2, 3, 4, 5
Affiliation
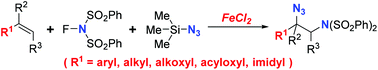
|
An iron-catalysed intermolecular vicinal aminoazidation of alkenes, using N-fluorobenzenesulfonimide (NFSI) and trimethylsilyl azide (TMSN3) as the imidating and azidating reagents, respectively, is described, which could potentially provide a valuable route toward diverse vicinal diamine derivatives of great significance in medicinal chemistry and organic synthesis. Such iron-catalysed aminative bisfunctionalization of alkenes with NFSI has not been reported yet. Comparing to previously employed copper or palladium catalysts, the iron catalyst, FeCl2, was demonstrated to be a good alternative for its comparable efficiency and broad alkene scope. Preliminary mechanistic study suggested that this iron-catalysed reaction is realized through radical processes.
中文翻译:

通过铁催化的NFSI参与的烯烃分子间氨基叠氮化†
描述了铁催化的烯烃间分子邻氨基叠氮化,分别使用N-氟苯磺酰亚胺(NFSI)和叠氮化三甲基硅烷基化试剂(TMSN 3),这可能为迈向大分子的各种邻位二胺衍生物提供有价值的途径在药物化学和有机合成中具有重要意义。尚未报道过这种铁催化的具有NFSI的烯烃的胺双官能化。与以前使用的铜或钯催化剂相比,铁催化剂FeCl 2被证明是可替代的催化剂,因为它具有可比的效率和宽泛的烯烃范围。初步的机理研究表明,这种铁催化的反应是通过自由基过程实现的。
更新日期:2018-04-11
中文翻译:

通过铁催化的NFSI参与的烯烃分子间氨基叠氮化†
描述了铁催化的烯烃间分子邻氨基叠氮化,分别使用N-氟苯磺酰亚胺(NFSI)和叠氮化三甲基硅烷基化试剂(TMSN 3),这可能为迈向大分子的各种邻位二胺衍生物提供有价值的途径在药物化学和有机合成中具有重要意义。尚未报道过这种铁催化的具有NFSI的烯烃的胺双官能化。与以前使用的铜或钯催化剂相比,铁催化剂FeCl 2被证明是可替代的催化剂,因为它具有可比的效率和宽泛的烯烃范围。初步的机理研究表明,这种铁催化的反应是通过自由基过程实现的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号