当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conformational Transition of Key Structural Features Involved in Activation of ALK Induced by Two Neuroblastoma Mutations and ATP Binding: Insight from Accelerated Molecular Dynamics Simulations
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-04-11 00:00:00 , DOI: 10.1021/acschemneuro.8b00105 Mu-Yang He 1 , Wei-Kang Li 1 , Qing-Chuan Zheng 1, 2 , Hong-Xing Zhang 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-04-11 00:00:00 , DOI: 10.1021/acschemneuro.8b00105 Mu-Yang He 1 , Wei-Kang Li 1 , Qing-Chuan Zheng 1, 2 , Hong-Xing Zhang 1
Affiliation
|
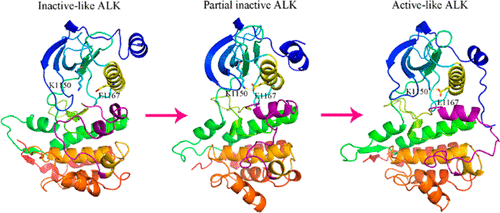
Deregulated kinase activity of anaplastic lymphoma kinase (ALK) has been observed to be implicated in the development of tumor progression. The activation mechanism of ALK is proposed to be similar to other receptor tyrosine kinases (RTKs), but the distinct static X-ray crystal conformation of ALK suggests its unique conformational transition. Herein, we have illustrated the dynamic conformational property of wild-type ALK as well as the kinase activation equilibrium variation induced by two neuroblastoma mutations (R1275Q and Y1278S) and ATP binding by performing enhanced sampling accelerated Molecular Dynamics (aMD) simulations. The results suggest that the wild-type ALK is mostly favored in the inactive state, whereas the mutations and ATP binding promote a clear shift toward the active-like conformation. The R1275Q mutant stabilizes the active conformation by rigidifying the αC-in conformation. The Y1278S mutant promotes activation at the expense of a π-stacking hydrophobic cluster, which plays a critical role in the stabilization of the inactive conformation of native ALK. ATP produces a more compact active site and thereby facilitates the activation of ALK. Taken together, these findings not only elucidate the diverse conformations in different ALKs but can also shed light on new strategies for protein engineering and structural-based drug design for ALK.
中文翻译:

涉及由两个神经母细胞瘤突变和ATP结合引起的ALK激活的关键结构特征的构象转变:加速的分子动力学模拟的见解。
已观察到间变性淋巴瘤激酶(ALK)的激酶活性失调与肿瘤进展的发展有关。提出ALK的激活机制与其他受体酪氨酸激酶(RTK)相似,但是ALK独特的静态X射线晶体构象表明其独特的构象转变。在这里,我们通过执行增强的采样加速分子动力学(aMD)模拟,说明了野生型ALK的动态构象特性,以及由两个神经母细胞瘤突变(R1275Q和Y1278S)和ATP结合诱导的激酶激活平衡变异。结果表明,野生型ALK在非活性状态下最受青睐,而突变和ATP结合则促进了向活性样构象的明显转变。R1275Q突变体通过刚性化αC-in构象来稳定活性构象。Y1278S突变体以π堆积疏水簇为代价来促进活化,这在稳定天然ALK的非活性构象方面起着关键作用。ATP产生一个更紧凑的活性位点,从而促进ALK的活化。综上所述,这些发现不仅阐明了不同ALK中的各种构象,而且还为蛋白质工程的新策略和ALK的基于结构的药物设计提供了新的思路。ATP产生一个更紧凑的活性位点,从而促进ALK的活化。综上所述,这些发现不仅阐明了不同ALK中的各种构象,而且还为蛋白质工程的新策略和ALK的基于结构的药物设计提供了新的思路。ATP产生一个更紧凑的活性位点,从而促进ALK的活化。综上所述,这些发现不仅阐明了不同ALK中的各种构象,而且还为蛋白质工程的新策略和ALK的基于结构的药物设计提供了新的思路。
更新日期:2018-04-11
中文翻译:

涉及由两个神经母细胞瘤突变和ATP结合引起的ALK激活的关键结构特征的构象转变:加速的分子动力学模拟的见解。
已观察到间变性淋巴瘤激酶(ALK)的激酶活性失调与肿瘤进展的发展有关。提出ALK的激活机制与其他受体酪氨酸激酶(RTK)相似,但是ALK独特的静态X射线晶体构象表明其独特的构象转变。在这里,我们通过执行增强的采样加速分子动力学(aMD)模拟,说明了野生型ALK的动态构象特性,以及由两个神经母细胞瘤突变(R1275Q和Y1278S)和ATP结合诱导的激酶激活平衡变异。结果表明,野生型ALK在非活性状态下最受青睐,而突变和ATP结合则促进了向活性样构象的明显转变。R1275Q突变体通过刚性化αC-in构象来稳定活性构象。Y1278S突变体以π堆积疏水簇为代价来促进活化,这在稳定天然ALK的非活性构象方面起着关键作用。ATP产生一个更紧凑的活性位点,从而促进ALK的活化。综上所述,这些发现不仅阐明了不同ALK中的各种构象,而且还为蛋白质工程的新策略和ALK的基于结构的药物设计提供了新的思路。ATP产生一个更紧凑的活性位点,从而促进ALK的活化。综上所述,这些发现不仅阐明了不同ALK中的各种构象,而且还为蛋白质工程的新策略和ALK的基于结构的药物设计提供了新的思路。ATP产生一个更紧凑的活性位点,从而促进ALK的活化。综上所述,这些发现不仅阐明了不同ALK中的各种构象,而且还为蛋白质工程的新策略和ALK的基于结构的药物设计提供了新的思路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号