当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DBU‐Catalyzed [3+3] and [3+2] Annulation Reactions of Azomethine Ylides with α‐Diazocarbonyls as N‐Terminal Electrophiles: Modular, Atom‐Economical Access to 1,2,4‐Triazine and 1,2,4‐Triazole Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-04-18 , DOI: 10.1002/adsc.201800030 Lu Zhang 1 , Jia-Jia Chen 1 , Sha-Sha Liu 2 , Yong-Xin Liang 1 , Yu-Long Zhao 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-04-18 , DOI: 10.1002/adsc.201800030 Lu Zhang 1 , Jia-Jia Chen 1 , Sha-Sha Liu 2 , Yong-Xin Liang 1 , Yu-Long Zhao 1
Affiliation
|
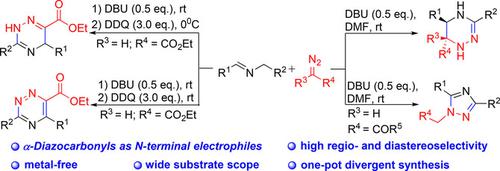
The DBU‐catalyzed [3+3] and [3+2] cyclization reactions of azomethine ylides with α‐diazocarbonyls as N‐terminal electrophiles have been developed. These reactions involve a sequential intermolecular nucleophilic addition/intramolecular cyclization/oxidation procedure. By the assembly of readily available starting materials, these transformations offer novel, highly efficient one‐pot syntheses of various functionalized 1,2,4‐triazine and 1,2,4‐triazole derivatives in an atom‐economical manner under ambient and metal‐free conditions in a high regio‐ and diastereoselective manner.
中文翻译:

DBU催化的偶氮甲萘啶与α-重氮羰基化合物作为N-端亲电体的[3 + 3]和[3 + 2]环化反应:通过模块化,原子经济方式获得1,2,4-三嗪和1,2,4-三唑衍生物
已经开发了DBU催化的甲亚胺基化物与α-重氮羰基作为N末端亲电试剂的[3 + 3]和[3 + 2]环化反应。这些反应涉及顺序的分子间亲核加成/分子内环化/氧化过程。通过容易获得的原料的组装,这些转变可在环境和金属环境下以原子经济的方式提供新颖,高效的一锅合成各种功能化的1,2,4-三嗪和1,2,4-三唑衍生物。自由区域,具有很高的区域选择性和非对映选择性。
更新日期:2018-04-18
中文翻译:

DBU催化的偶氮甲萘啶与α-重氮羰基化合物作为N-端亲电体的[3 + 3]和[3 + 2]环化反应:通过模块化,原子经济方式获得1,2,4-三嗪和1,2,4-三唑衍生物
已经开发了DBU催化的甲亚胺基化物与α-重氮羰基作为N末端亲电试剂的[3 + 3]和[3 + 2]环化反应。这些反应涉及顺序的分子间亲核加成/分子内环化/氧化过程。通过容易获得的原料的组装,这些转变可在环境和金属环境下以原子经济的方式提供新颖,高效的一锅合成各种功能化的1,2,4-三嗪和1,2,4-三唑衍生物。自由区域,具有很高的区域选择性和非对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号