当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium‐Catalyzed Tandem Cyclization of Benzoylacetonitriles with Diazo Compounds Leading to Substituted Naphtho[1,8‐bc]pyrans by Sequential C−H Functionalization
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-04-19 , DOI: 10.1002/adsc.201800149 Kelu Yan 1 , Bin Li 1 , Baiquan Wang 1, 2, 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-04-19 , DOI: 10.1002/adsc.201800149 Kelu Yan 1 , Bin Li 1 , Baiquan Wang 1, 2, 3
Affiliation
|
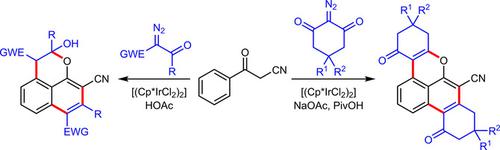
The cascade annulation reactions of benzoylacetonitriles with diazo compounds proceed efficiently in the presence of an iridium catalyst to give substituted naphtho[1,8‐bc]pyrans by sequential cleavage of C(sp2)−H/C(sp3)−H and C(sp2)−H/O−H bonds. Interestingly, the reactions involving cyclic diazo compounds and open‐chain diazo compounds lead to different types of naphtho[1,8‐bc]pyrans. Most products are obtained in moderate to good yields with a broad range of substrates.
中文翻译:

铱催化串联苯甲酰乙腈与重氮化合物的连续串联环化反应,通过连续CH官能团官能化取代萘并[1,8-bc]吡喃
benzoylacetonitriles与重氮化合物级联环反应在铱催化剂的存在下有效地进行,得到取代的萘并[1,8- BC ]通过C(的顺序裂解吡喃SP 2)-H / C(SP 3)-H和C(sp 2)-H / OH键。有趣的是,涉及环状重氮化合物和开链重氮化合物的反应会导致不同类型的萘并[1,8- bc ]吡喃。大多数产品都是以中等到良好的收率获得的,并具有广泛的底物。
更新日期:2018-04-19
中文翻译:

铱催化串联苯甲酰乙腈与重氮化合物的连续串联环化反应,通过连续CH官能团官能化取代萘并[1,8-bc]吡喃
benzoylacetonitriles与重氮化合物级联环反应在铱催化剂的存在下有效地进行,得到取代的萘并[1,8- BC ]通过C(的顺序裂解吡喃SP 2)-H / C(SP 3)-H和C(sp 2)-H / OH键。有趣的是,涉及环状重氮化合物和开链重氮化合物的反应会导致不同类型的萘并[1,8- bc ]吡喃。大多数产品都是以中等到良好的收率获得的,并具有广泛的底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号