当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protecting group free radical C–H trifluoromethylation of peptides†
Chemical Science ( IF 7.6 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1039/c8sc00368h Naoko Ichiishi 1 , John P Caldwell 2 , Melissa Lin 3 , Wendy Zhong 3 , Xiaohong Zhu 2 , Eric Streckfuss 4 , Hai-Young Kim 3 , Craig A Parish 5 , Shane W Krska 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1039/c8sc00368h Naoko Ichiishi 1 , John P Caldwell 2 , Melissa Lin 3 , Wendy Zhong 3 , Xiaohong Zhu 2 , Eric Streckfuss 4 , Hai-Young Kim 3 , Craig A Parish 5 , Shane W Krska 1
Affiliation
|
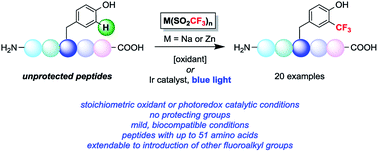
Two radical-based approaches have been developed to effect the trifluoromethylation of aryl C–H bonds in native peptides either using stoichiometric oxidant or visible light photoredox catalysis. The reported methods are able to derivatize tyrosine and tryptophan sidechains under biocompatible conditions, and a number of examples are reported involving fully unprotected peptides with up to 51 amino acids. The development of this chemistry adds to the growing array of chemical methods for selectively modifying amino acid residues in the context of complex peptides. The direct incorporation of trifluoromethyl groups into biopolymers enables the study of a range of biological and biochemical systems, and preliminary results indicate this method can be extended to the incorporation of other fluoroalkyl groups for bioconjugation applications.
中文翻译:

肽的保护基团自由基 C–H 三氟甲基化†
已经开发了两种基于自由基的方法,使用化学计量氧化剂或可见光光氧化还原催化来实现天然肽中芳基 C-H 键的三氟甲基化。所报道的方法能够在生物相容性条件下衍生化酪氨酸和色氨酸侧链,并且报道了许多涉及具有多达51个氨基酸的完全未受保护的肽的例子。这种化学的发展增加了越来越多的化学方法,用于选择性修饰复杂肽中的氨基酸残基。将三氟甲基直接掺入生物聚合物中可以研究一系列生物和生化系统,初步结果表明该方法可以扩展到掺入其他氟烷基用于生物共轭应用。
更新日期:2018-04-10
中文翻译:

肽的保护基团自由基 C–H 三氟甲基化†
已经开发了两种基于自由基的方法,使用化学计量氧化剂或可见光光氧化还原催化来实现天然肽中芳基 C-H 键的三氟甲基化。所报道的方法能够在生物相容性条件下衍生化酪氨酸和色氨酸侧链,并且报道了许多涉及具有多达51个氨基酸的完全未受保护的肽的例子。这种化学的发展增加了越来越多的化学方法,用于选择性修饰复杂肽中的氨基酸残基。将三氟甲基直接掺入生物聚合物中可以研究一系列生物和生化系统,初步结果表明该方法可以扩展到掺入其他氟烷基用于生物共轭应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号