当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Transformation of CO2 to CO at a Single Nickel Center
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1021/acs.accounts.7b00634 Changho Yoo 1 , Yeong-Eun Kim 1 , Yunho Lee 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1021/acs.accounts.7b00634 Changho Yoo 1 , Yeong-Eun Kim 1 , Yunho Lee 1
Affiliation
|
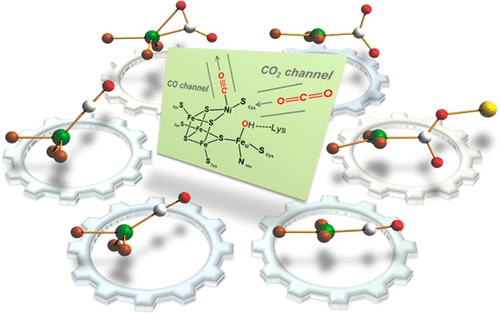
Carbon dioxide conversion mediated by transition metal complexes continues to attract much attention because of its future potential utilization as a nontoxic and inexpensive C1 source for the chemical industry. Given the presence of nickel in natural systems that allow for extremely efficient catalysis, albeit in an Fe cluster arrangement, studies that focus on selective CO2 conversion with synthetic nickel species are currently of considerable interest in our group. In this Account, the selective conversion of CO2 to carbon monoxide occurring at a single nickel center is discussed. The chemistry is based on a series of related nickel pincer complexes with attention to the uniqueness of the coordination geometry, which is crucial in allowing for particular reactivity toward CO2. Our research is inspired by the efficient enzymatic CO2 catalysis occurring at the active site of carbon monoxide dehydrogenase. Since the binding and reactivity toward CO2 are controlled in part by the geometry of a L3Ni scaffold, we have explored the chemistry of low-valent nickel supported by PPMeP and PNP ligands, in which a pseudotetrahedral or square-planar geometry is accommodated. Two isolated nickel–CO2 adducts, (PPMeP)Ni(η2-CO2-κC) (2) and {Na(12-C-4)2}{(PNP)Ni(η1-CO2-κC)} (7), clearly demonstrate that the geometry of the nickel ion is crucial in the binding of CO2 and its level of activation. In the case of a square-planar nickel center supported by a PNP ligand, a series of bimetallic metallacarboxylate Ni−μ-CO2-κC,O–M species (M = H, Na, Ni, Fe) were synthesized, and their structural features and reactivity were studied. Protonation cleaves the C–O bond, resulting in the formation of a nickel(II) monocarbonyl complex. By sequential reduction, the corresponding mono- and zero-valent Ni–CO species were produced. The reactivities of three nickel carbonyl species toward various iodoalkanes and CO2 were explored to address whether their corresponding reactivities could be controlled by the number of valence d electrons. In particular, a (PNP)Ni(0)–CO species (13) shows immediate reactivity toward CO2 but displays multiple product formation. By incorporation of a −CMe2– bridging unit, a structurally rigidified acriPNP ligand was newly designed and produced. This ligand modification was successful in preparing the T-shaped nickel(I) metalloradical species 9 exhibiting open-shell reactivity due to the sterically exposed nickel center possessing a half-filled dx2–y2 orbital. More importantly, the selective addition of CO2 to a nickel(0)–CO species was enabled to afford a nickel(II)–carboxylate species (22) with the expulsion of CO(g). Finally, the (acriPNP)Ni system provides a synthetic cycle in the study of the selective conversion of CO2 to CO that involves two-electron reduction of Ni–CO followed by the direct addition of CO2 to release the coordinated CO ligand.
中文翻译:

在单个镍中心将CO 2选择性转化为CO
由于过渡金属络合物作为化学工业的无毒且廉价的C1来源,其潜在的未来利用前景,因此过渡金属络合物介导的二氧化碳转化一直备受关注。考虑到镍在自然系统中的存在,即使是铁簇排列,也能实现极其高效的催化作用,目前研究集中在利用合成镍物质进行选择性CO 2转化的研究上。在该报告中,讨论了在单个镍中心发生的CO 2到一氧化碳的选择性转化。化学基于一系列相关的镍钳配合物,并注意配位几何结构的独特性,这对于允许特定的对CO的反应性至关重要2。我们的研究受到在一氧化碳脱氢酶活性位点发生的高效酶促CO 2催化的启发。由于对CO 2的结合和反应性部分受L 3 Ni支架的几何形状控制,因此我们探索了由PP Me P和PNP配体支撑的低价镍的化学性质,其中伪四面体或方形结构被容纳。两个隔离的镍-CO 2加合物,(PP我P)的Ni(η 2 -CO 2 -κ Ç)(2)和{的Na(12-C-4)2 } {(PNP)的Ni(η 1 -CO 2 -κC)}(7)清楚地表明,镍离子的几何形状对于CO 2的结合及其活化程度至关重要。在由PNP配体,系列双金属metallacarboxylate镍μ-CO的支撑的正方形平面镍中心的情况下,2 -κ Ç,ö -M物种(M = H,钠,镍,铁)的合成,并研究了它们的结构特征和反应性。质子化裂解了C–O键,从而形成了镍(II)单羰基配合物。通过顺序还原,产生了相应的一价和零价Ni-CO物种。三种羰基镍物种对各种碘代烷烃和CO 2的反应性为了解决它们相应的反应性是否可以被价电子的数量控制而进行了探索。特别是(PNP)Ni(0)-CO物种(13)显示出对CO 2的立即反应性,但显示出多种产物形成。通过掺入-CMe 2-桥联单元,新设计并生产了结构刚性的acri PNP配体。这种配体修饰成功地制备了T形镍(I)金属原子种类9,这是由于在空间上暴露的镍中心具有半填充的d x 2 - y 2轨道而表现出的开壳反应性。更重要的是,选择性添加一氧化碳镍(0)–CO物种2能够通过驱逐CO(g)来提供镍(II)–羧酸盐物种(22)。最终,(acri PNP)Ni系统为CO 2选择性转化为CO的研究提供了一个合成循环,该过程涉及Ni-CO的两电子还原,然后直接添加CO 2以释放出配位的CO配体。
更新日期:2018-04-10
中文翻译:

在单个镍中心将CO 2选择性转化为CO
由于过渡金属络合物作为化学工业的无毒且廉价的C1来源,其潜在的未来利用前景,因此过渡金属络合物介导的二氧化碳转化一直备受关注。考虑到镍在自然系统中的存在,即使是铁簇排列,也能实现极其高效的催化作用,目前研究集中在利用合成镍物质进行选择性CO 2转化的研究上。在该报告中,讨论了在单个镍中心发生的CO 2到一氧化碳的选择性转化。化学基于一系列相关的镍钳配合物,并注意配位几何结构的独特性,这对于允许特定的对CO的反应性至关重要2。我们的研究受到在一氧化碳脱氢酶活性位点发生的高效酶促CO 2催化的启发。由于对CO 2的结合和反应性部分受L 3 Ni支架的几何形状控制,因此我们探索了由PP Me P和PNP配体支撑的低价镍的化学性质,其中伪四面体或方形结构被容纳。两个隔离的镍-CO 2加合物,(PP我P)的Ni(η 2 -CO 2 -κ Ç)(2)和{的Na(12-C-4)2 } {(PNP)的Ni(η 1 -CO 2 -κC)}(7)清楚地表明,镍离子的几何形状对于CO 2的结合及其活化程度至关重要。在由PNP配体,系列双金属metallacarboxylate镍μ-CO的支撑的正方形平面镍中心的情况下,2 -κ Ç,ö -M物种(M = H,钠,镍,铁)的合成,并研究了它们的结构特征和反应性。质子化裂解了C–O键,从而形成了镍(II)单羰基配合物。通过顺序还原,产生了相应的一价和零价Ni-CO物种。三种羰基镍物种对各种碘代烷烃和CO 2的反应性为了解决它们相应的反应性是否可以被价电子的数量控制而进行了探索。特别是(PNP)Ni(0)-CO物种(13)显示出对CO 2的立即反应性,但显示出多种产物形成。通过掺入-CMe 2-桥联单元,新设计并生产了结构刚性的acri PNP配体。这种配体修饰成功地制备了T形镍(I)金属原子种类9,这是由于在空间上暴露的镍中心具有半填充的d x 2 - y 2轨道而表现出的开壳反应性。更重要的是,选择性添加一氧化碳镍(0)–CO物种2能够通过驱逐CO(g)来提供镍(II)–羧酸盐物种(22)。最终,(acri PNP)Ni系统为CO 2选择性转化为CO的研究提供了一个合成循环,该过程涉及Ni-CO的两电子还原,然后直接添加CO 2以释放出配位的CO配体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号