当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CysLTR1 Blockage Ameliorates Liver Injury Caused by Aluminum-Overload via PI3K/AKT/mTOR-Mediated Autophagy Activation in Vivo and in Vitro
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00121 Congli Hu 1 , Junqing Yang 1 , Qin He 2 , Ying Luo 1 , Zhihao Chen 1 , Lu Yang 1 , Honggang Yi 1 , Huan Li 1 , Hui Xia 1 , Dongzhi Ran 1 , Yang Yang 1 , Jiahua Zhang 1 , Yuke Li 1 , Hong Wang 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00121 Congli Hu 1 , Junqing Yang 1 , Qin He 2 , Ying Luo 1 , Zhihao Chen 1 , Lu Yang 1 , Honggang Yi 1 , Huan Li 1 , Hui Xia 1 , Dongzhi Ran 1 , Yang Yang 1 , Jiahua Zhang 1 , Yuke Li 1 , Hong Wang 1
Affiliation
|
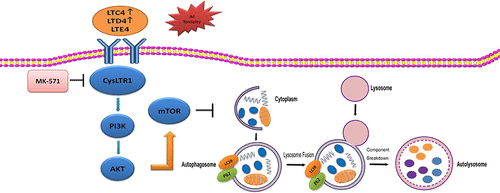
Aluminum (Al) is a trivalent cation that can accumulate in animal organs, especially in the liver. We previously demonstrated that Al-overload could induce liver morphologic aberrations and dysfunction. However, the molecular mechanism underlying liver injury caused by Al-overload still remains unknown. In the present study, we investigated the relationship between leukotrienes receptors and the PI3K/AKT/mTOR pathway in Al-induced liver injury in vivo and in vitro. We demonstrated that Al-overload significantly increased the protein expression levels of CysLTR1, PI3K, AKT, mTOR, and p62, while significantly decreasing the LC3BII protein levels in rat liver; thus, suggesting that the autophagy process was inhibited in Al-overloaded rat liver. In addition, MK-571, an inhibitor of CysLTR1, effectively protected the human hepatocyte L02 cells against injury caused by Al exposure. Moreover, CysLTR1 blockage could significantly down-regulate the PI3K/AKT/mTOR pathway and activate autophagy. The effect of MK-571 on cell viability was abolished by the treatment with the autophagy inhibitor (wortmannin) but not with the autophagy agonist (rapamycin). Taken together, our results indicated that the blockage of the leukotriene receptor of CysLTR1 promotes autophagy and further reduces hepatocyte death through the PI3K/AKT/mTOR pathway inhibition. CysLTR1 thus could represent a potential target for the new drug development for chronic noninfective liver injury.
中文翻译:

CysLTR1阻滞减轻了体内和体外PI3K / AKT / mTOR介导的自噬激活引起的铝超载引起的肝损伤。
铝(Al)是三价阳离子,可以在动物器官中,尤其是肝脏中积累。先前我们证明了铝超负荷可引起肝脏形态畸变和功能障碍。然而,由铝超载引起的肝损伤的分子机制仍是未知的。在本研究中,我们在体内和体外研究了白三烯受体与PI介导的PI3K / AKT / mTOR途径之间的关系。我们证明了铝超负荷显着增加了CysLTR1,PI3K,AKT,mTOR和p62的蛋白质表达水平,同时显着降低了大鼠肝脏中LC3BII的蛋白质水平;因此,这表明自噬过程在铝超载大鼠肝脏中受到抑制。另外,CysLTR1的抑制剂MK-571 有效保护人肝细胞L02细胞免受Al暴露造成的伤害。而且,CysLTR1的阻滞可能显着下调PI3K / AKT / mTOR通路并激活自噬。用自噬抑制剂(渥曼青霉素)治疗,但未用自噬激动剂(雷帕霉素)治疗,从而消除了MK-571对细胞活力的影响。两者合计,我们的结果表明CysLTR1的白三烯受体的阻断促进自噬并通过PI3K / AKT / mTOR途径抑制进一步减少肝细胞死亡。因此,CysLTR1可能代表了针对慢性非感染性肝损伤的新药开发的潜在目标。用自噬抑制剂(渥曼青霉素)治疗,但未用自噬激动剂(雷帕霉素)治疗,从而消除了MK-571对细胞活力的影响。两者合计,我们的结果表明CysLTR1的白三烯受体的阻断促进自噬并通过PI3K / AKT / mTOR途径抑制进一步减少肝细胞死亡。因此,CysLTR1可能代表了针对慢性非感染性肝损伤的新药开发的潜在目标。用自噬抑制剂(渥曼青霉素)治疗,但未用自噬激动剂(雷帕霉素)治疗,从而消除了MK-571对细胞活力的影响。两者合计,我们的结果表明CysLTR1的白三烯受体的阻断促进自噬并通过PI3K / AKT / mTOR途径抑制进一步减少肝细胞死亡。因此,CysLTR1可能代表了针对慢性非感染性肝损伤的新药开发的潜在目标。
更新日期:2018-04-10
中文翻译:

CysLTR1阻滞减轻了体内和体外PI3K / AKT / mTOR介导的自噬激活引起的铝超载引起的肝损伤。
铝(Al)是三价阳离子,可以在动物器官中,尤其是肝脏中积累。先前我们证明了铝超负荷可引起肝脏形态畸变和功能障碍。然而,由铝超载引起的肝损伤的分子机制仍是未知的。在本研究中,我们在体内和体外研究了白三烯受体与PI介导的PI3K / AKT / mTOR途径之间的关系。我们证明了铝超负荷显着增加了CysLTR1,PI3K,AKT,mTOR和p62的蛋白质表达水平,同时显着降低了大鼠肝脏中LC3BII的蛋白质水平;因此,这表明自噬过程在铝超载大鼠肝脏中受到抑制。另外,CysLTR1的抑制剂MK-571 有效保护人肝细胞L02细胞免受Al暴露造成的伤害。而且,CysLTR1的阻滞可能显着下调PI3K / AKT / mTOR通路并激活自噬。用自噬抑制剂(渥曼青霉素)治疗,但未用自噬激动剂(雷帕霉素)治疗,从而消除了MK-571对细胞活力的影响。两者合计,我们的结果表明CysLTR1的白三烯受体的阻断促进自噬并通过PI3K / AKT / mTOR途径抑制进一步减少肝细胞死亡。因此,CysLTR1可能代表了针对慢性非感染性肝损伤的新药开发的潜在目标。用自噬抑制剂(渥曼青霉素)治疗,但未用自噬激动剂(雷帕霉素)治疗,从而消除了MK-571对细胞活力的影响。两者合计,我们的结果表明CysLTR1的白三烯受体的阻断促进自噬并通过PI3K / AKT / mTOR途径抑制进一步减少肝细胞死亡。因此,CysLTR1可能代表了针对慢性非感染性肝损伤的新药开发的潜在目标。用自噬抑制剂(渥曼青霉素)治疗,但未用自噬激动剂(雷帕霉素)治疗,从而消除了MK-571对细胞活力的影响。两者合计,我们的结果表明CysLTR1的白三烯受体的阻断促进自噬并通过PI3K / AKT / mTOR途径抑制进一步减少肝细胞死亡。因此,CysLTR1可能代表了针对慢性非感染性肝损伤的新药开发的潜在目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号