Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2018-04-11 , DOI: 10.1016/j.molliq.2018.04.031 Mohd. Azfar Shaida , R.K. Dutta , A.K. Sen
|
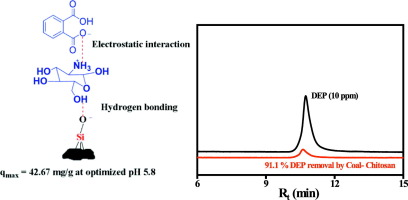
Phthalic acid esters or phthalates in wastewater are priority pollutants and demand efficient method for remediation. Here we present a novel utilization of low grade coal, which is otherwise a waste, modified by chitosan as adsorbent for removal of diethyl phthalate (DEP) via adsorption. A low carbon and high silicate content coal was chosen, characterized by CHN analyzer, energy dispersive X-ray analysis and by X-ray diffraction. A composite of mineral rich coal and chitosan is prepared via electrostatic interaction and hydrogen bonding between the hydroxyl groups of chitosan and the silicate group of coal; confirmed by Fourier transformed infrared spectroscopy, thermogravimetry analysis and zeta potential measurements. An optimized 9:1 weight ratio of coal-chitosan composite exhibited a positive zeta potential value in aqueous medium and corresponded to 91.1% DEP adsorption at an optimized condition e.g., pH 5.8, adsorbent dose of 4 mg/mL and contact time of 4 h. The adsorption is thermodynamically favorable and followed pseudo-second order kinetics, and attributable to chemisorption process, is attributed to electrostatic interaction and hydrogen bonding between the DEP and the functional groups in the composite. The adsorption isotherm data was best fitted non-linearly by Sips isotherm model, which is consistent with Freundlich adsorption isotherm for lower concentration of DEP (5 to 100 mg/L), while Langmuir adsorption was favored for adsorption of higher DEP concentrations (100 to 400 mg/L). The qmax was 42.67 mg/g, which is a significant improvement in DEP sorption from aqueous medium among the readily degradable adsorbents reported so far. The practical application of coal-chitosan composite as adsorbent has been demonstrated by its re-usability and ability for sorption of DEP from spiked municipal wastewater, supported by decrease in chemical oxygen demand value of treated wastewater.
中文翻译:

壳聚糖改性富矿废煤吸附脱除邻苯二甲酸二乙酯
废水中的邻苯二甲酸酯或邻苯二甲酸酯是主要污染物,需要有效的补救方法。在这里,我们介绍了一种新的利用低品位煤的方法,它是一种由壳聚糖改性的吸附剂,它是一种废物,可通过吸附去除邻苯二甲酸二乙酯(DEP)。选择了低碳高硅酸盐含量的煤,通过CHN分析仪,能量色散X射线分析和X射线衍射对其进行了表征。通过壳聚糖的羟基与煤的硅酸盐基团之间的静电相互作用和氢键结合,制备出富含矿物质的煤和壳聚糖的复合物。通过傅立叶变换红外光谱,热重分析和Zeta电位测量得到证实。优化的9:1重量比的煤-壳聚糖复合材料在水性介质中显示出正的Zeta电位值,并且在优化的条件(例如pH 5.8),吸附剂剂量为4 mg / mL和接触时间为4 h时,相当于91.1%的DEP吸附。吸附在热力学上是有利的,并且遵循伪二级动力学,并且归因于化学吸附过程,这归因于DEP与复合材料中官能团之间的静电相互作用和氢键。吸附等温线数据最好通过Sips等温线模型进行非线性拟合,这与较低浓度DEP(5至100 mg / L)的Freundlich吸附等温线一致,而Langmuir吸附有利于较高浓度DEP(100至100 mg / L)的吸附。 400 mg / L)。这 吸附剂剂量为4 mg / mL,接触时间为4 h。吸附在热力学上是有利的,并且遵循伪二级动力学,并且归因于化学吸附过程,这归因于DEP与复合材料中官能团之间的静电相互作用和氢键。吸附等温线数据最好通过Sips等温线模型进行非线性拟合,这与较低浓度DEP(5至100 mg / L)的Freundlich吸附等温线一致,而Langmuir吸附有利于较高浓度DEP(100至100 mg / L)的吸附。 400 mg / L)。这 吸附剂剂量为4 mg / mL,接触时间为4 h。吸附在热力学上是有利的,并且遵循伪二级动力学,并且归因于化学吸附过程,这归因于DEP与复合材料中官能团之间的静电相互作用和氢键。吸附等温线数据最好通过Sips等温线模型进行非线性拟合,这与较低浓度DEP(5至100 mg / L)的Freundlich吸附等温线一致,而Langmuir吸附有利于较高浓度DEP(100至100 mg / L)的吸附。 400 mg / L)。这 这归因于DEP和复合材料中官能团之间的静电相互作用和氢键。吸附等温线数据最好通过Sips等温线模型非线性拟合,这与低浓度DEP(5至100 mg / L)的Freundlich吸附等温线一致,而Langmuir吸附有利于较高DEP浓度(100至100 400 mg / L)。这 这归因于DEP和复合材料中官能团之间的静电相互作用和氢键。吸附等温线数据最好通过Sips等温线模型进行非线性拟合,这与较低浓度DEP(5至100 mg / L)的Freundlich吸附等温线一致,而Langmuir吸附有利于较高浓度DEP(100至100 mg / L)的吸附。 400 mg / L)。这q max为42.67 mg / g,这是迄今为止报道的易降解吸附剂中水介质对DEP吸附的显着改善。煤-壳聚糖复合材料作为吸附剂的实际应用已经证明了其的可重复使用性和对加标市政废水中DEP的吸附能力,同时得到了处理后废水化学需氧量的降低的支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号