当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Drimane Sesquiterpenoids Noncompetitively Inhibit Human α4β2 Nicotinic Acetylcholine Receptors with Higher Potency Compared to Human α3β4 and α7 Subtypes
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00893 Hugo R. Arias 1 , Dominik Feuerbach 2 , Bernd Schmidt 3 , Matthias Heydenreich 3 , Cristian Paz 4 , Marcelo O. Ortells 5
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00893 Hugo R. Arias 1 , Dominik Feuerbach 2 , Bernd Schmidt 3 , Matthias Heydenreich 3 , Cristian Paz 4 , Marcelo O. Ortells 5
Affiliation
|
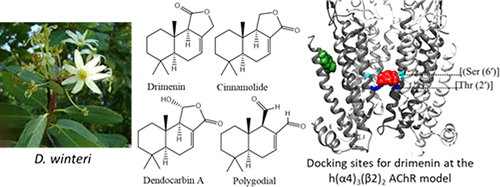
The drimane sesquiterpenoids drimenin, cinnamolide, dendocarbin A, and polygodial were purified from the Canelo tree (Drimys winteri) and chemically characterized by spectroscopic methods. The pharmacological activity of these natural compounds were determined on hα4β2, hα3β4, and hα7 nicotinic acetylcholine receptors (AChRs) by Ca2+ influx measurements. The results established that drimane sesquiterpenoids inhibit AChRs with the following selectivity: hα4β2 > hα3β4 > hα7. In the case of hα4β2 AChRs, the following potency rank order was determined (IC50’s in μM): drimenin (0.97 ± 0.35) > cinnamolide (1.57 ± 0.36) > polygodial (62.5 ± 19.9) ≫ dendocarbin A (no activity). To determine putative structural features underlying the differences in inhibitory potency at hα4β2 AChRs, additional structure–activity relationship and molecular docking experiments were performed. The Ca2+ influx and structural results supported a noncompetitive mechanism of inhibition, where drimenin interacted with luminal and nonluminal (TMD-β2 intrasubunit) sites. The structure–activity relationship results, i.e., the lower the ligand polarity, the higher the inhibitory potency, supported the nonluminal interaction. Ligand binding to both sites might inhibit the hα4β2 AChR by a cooperative mechanism, as shown experimentally (nH > 1). Drimenin could be used as a molecular scaffold for the development of more potent inhibitors with higher selectivity for the hα4β2 AChR.
中文翻译:

与人α3β4和α7亚型相比,地烷的倍半萜类化合物非竞争性地抑制人α4β2烟碱乙酰胆碱受体。
从卡内洛树(Drimys winteri)中纯化了树烯倍半萜类树烯drimenin,肉桂酸酯,树突卡宾A和多角体,并通过光谱法对其进行化学表征。这些天然化合物的药理活性通过Ca 2+内流测量在hα4β2,hα3β4和hα7烟碱乙酰胆碱受体(AChRs)上确定。结果证实,drimane倍半萜能以下列选择性抑制AChR:hα4β2>hα3β4>hα7。对于hα4β2AChRs,确定以下效价等级顺序(IC 50的单位为μM):drimenin(0.97±0.35)>肉桂酸酯(1.57±0.36)> polygodial(62.5±19.9)≫ dendocarbin A(无活性)。为了确定在hα4β2AChRs抑制能力上的差异所基于的推定结构特征,进行了额外的结构-活性关系和分子对接实验。Ca 2+的涌入和结构结果支持了一种非竞争性的抑制机制,其中drimenin与腔和非腔(TMD-β2内亚基)位点相互作用。结构与活性之间的关系得到了结果,即配体极性越低,抑制力越高,支持了非内腔相互作用。如实验所示(n H> 1)。Drimenin可以用作分子支架,用于开发对hα4β2AChR具有更高选择性的更有效的抑制剂。
更新日期:2018-04-10
中文翻译:

与人α3β4和α7亚型相比,地烷的倍半萜类化合物非竞争性地抑制人α4β2烟碱乙酰胆碱受体。
从卡内洛树(Drimys winteri)中纯化了树烯倍半萜类树烯drimenin,肉桂酸酯,树突卡宾A和多角体,并通过光谱法对其进行化学表征。这些天然化合物的药理活性通过Ca 2+内流测量在hα4β2,hα3β4和hα7烟碱乙酰胆碱受体(AChRs)上确定。结果证实,drimane倍半萜能以下列选择性抑制AChR:hα4β2>hα3β4>hα7。对于hα4β2AChRs,确定以下效价等级顺序(IC 50的单位为μM):drimenin(0.97±0.35)>肉桂酸酯(1.57±0.36)> polygodial(62.5±19.9)≫ dendocarbin A(无活性)。为了确定在hα4β2AChRs抑制能力上的差异所基于的推定结构特征,进行了额外的结构-活性关系和分子对接实验。Ca 2+的涌入和结构结果支持了一种非竞争性的抑制机制,其中drimenin与腔和非腔(TMD-β2内亚基)位点相互作用。结构与活性之间的关系得到了结果,即配体极性越低,抑制力越高,支持了非内腔相互作用。如实验所示(n H> 1)。Drimenin可以用作分子支架,用于开发对hα4β2AChR具有更高选择性的更有效的抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号